Effects of Short-Term Exposure to Lithium on Antiapoptotic Bcl-xL Protein Expression in Cortex and Hippocampus of Rats after Acute Stress
N. N. Dygalo1,2*, A. V. Bannova1, E. V. Sukhareva1,2, G. T. Shishkina1, K. A. Ayriyants1,2, and T. S. Kalinina1,2
1Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia2Novosibirsk State University, 630090 Novosibirsk, Russia; E-mail: dygalo@bionet.nsc.ru
* To whom correspondence should be addressed.
Received October 19, 2016
The antiapoptotic protein Bcl-xL is involved in development of neurobiological resilience to stress; hence, the possibility of use of psychotropic drugs to increase its expression in brain in response to stress is of considerable interest. Lithium is a neurotropic drug widely used in psychiatry. In work, we studied effects of lithium administration (for 2 or 7 days) on the expression of Bcl-xL mRNA and protein in the hippocampi and cortices of rats subjected to stress that induced depression-like behavior in the animals. In contrast to the brain-derived neurotrophic factor (BDNF), whose expression decreased in the hippocampus in response to acute stress, stress increased the level of Bcl-xL mRNA in the hippocampus, but decreased it in the frontal cortex. Treatment of stressed animals with lithium for 2 or 7 days increased Bcl-xL protein levels 1.5-fold in the hippocampus, but it decreased them in the cortex. Therefore, Bcl-xL expression in the brain can be modulated by both stress and psychotropic drugs, and the effects of these factors are brain region-specific: both stress exposure and lithium administration activated Bcl-xL expression in the hippocampus and suppressed it in the frontal cortex. The activation of Bcl-xL expression in the hippocampus by lithium, demonstrated for the first time in this study, suggests an important role of this protein in the therapeutic effects of lithium in the treatment of stress-induced psychoemotional disorders.
KEY WORDS: Bcl-xL, BDNF, lithium chloride, stress, frontal cortex, hippocampus, gene expressionDOI: 10.1134/S0006297917030130
Dysregulation of brain neuronal plasticity caused by exposure to stress is believed to be one of the reasons for the development of depression [1]. Brain-derived neurotrophic factor (BDNF) regulates neurogenesis and cell survival and plays an important role in the structural effects of stress in the brain. Some of BDNF neuroprotective functions are mediated by antiapoptotic proteins such as Bcl-xL [2]. Apart from its main function, which is protection of all types of cells against programmed cell death, Bcl-xL also regulates the number, size, and activity of synapses, release of neurotransmitters, synaptic vesicle recycling, and spontaneous and induced synaptic responses [3-6]. Bcl-xL expression can rapidly change in response to increase in neuronal activity [7]. Such changes in expression levels are typical for proteins involved in neuroplasticity processes that can change the structure and properties of neurons upon their activation. Similar properties of Bcl-xL might explain the observed correlations between increased levels of Bcl-xL expression and psychoemotional and neurochemical resistance of brain to short-term stress or to the action of antidepressants [8-11]. This raises the question whether Bcl-xL expression in brain can be activated by psychotropic agents under stress exposure. Lithium salts have attracted considerable interest as potential neuroprotective agents. These compounds are already widely used as mood stabilizers in the therapy of bipolar disorders [12, 13]. It was found that lithium can also alter the expression of BDNF and of the antiapoptotic Bcl-2 protein in brain [14, 15]. Bcl-2 is the best-studied antiapoptotic protein; it is similar to Bcl-xL in its ability to protect cells against apoptosis. However, there is still no evidence that Bcl-2 can affect the stability of synaptic connections, neurotransmitter release, or response to neuronal firing. Considering the role of Bcl-xL in neuroplasticity [3-7], it seemed important to investigate the effects of lithium on the expression of this protein in brain to understand the mechanisms of action of compounds of this class or to search for new therapeutic targets. In this study, we evaluated the effects of short-term (2- and 7-day) administration of lithium (LiCl) on the expression of Bcl-xL mRNA and protein in the cortex and hippocampus of adult rats subjected to stress that induced depression-like behavior. For comparison, we estimated the changes in the expression of the classical neuroprotective factor BDNF.
MATERIALS AND METHODS
Adult male Wistar rats of four groups (10 animals per group) were used in experiments. Animals of the first group received no injections. Animals of the second group were injected peritoneally with physiological saline for 7 consecutive days. Animals of the third group were injected with physiological saline for five consecutive days and then with LiCl (84 mg/kg body weight, i. p.) for 2 days. Animals of the fourth group were injected peritoneally with the same dose of lithium chloride for 7 consecutive days. The lithium chloride dose used is known to provide therapeutic levels of lithium in human brain and to cause behavioral effects in experimental animals. On days 6 and 7, rats were exposed to stress 2 h after injections. The stress exposure included two sessions of forced swimming: 15-min pretest session and 5-min test session. Two hours after the last session, i.e. after the animals developed the typical depression-like behavior, they were sacrificed on ice. Frontal cortices and hippocampi were rapidly isolated and stored in liquid nitrogen.
Total RNA was isolated from the brain tissue samples by a one-step guanidine isothiocyanate method. The mRNAs for the bdnf and bcl-xL genes were quantified by real-time qPCR with the actb mRNA as a calibrator using a set of TaqMan® Gene Expression Assay primers/probes (Rn02531967_s1 for bdnf, Rn00437783_m1 for bcl-xL, and Rn00667869_m1 for actb; Applied Biosystems, USA) in an ABI VIIA™ 7 Real-Time PCR System (Applied Biosystems). The mRNA contents were calculated using the ΔΔCt method.
To estimate the levels of Bcl-xL protein by Western blot analysis, hippocampal and cortical tissue samples were homogenized in lysis buffer containing 150 mM NaCl, 50 mM Tris, 1% Triton X-100, and protease inhibitors (2 mM PMSF, 2 µg/ml leupeptin, 2 µg/ml pepstatin, and 2 µg/ml aprotinin). Protein samples (50 µg) were fractionated by electrophoresis in 12% polyacrylamide gel with sodium dodecyl sulfate in a Mini-Protean 3 Dodeca Cell (Bio-Rad, USA) and transferred onto 0.45 µm nitrocellulose membrane with a Trans-Blot system (Bio-Rad). The proteins were stained with primary anti-Bcl-xL rabbit monoclonal antibodies (dilution 1 : 500; Cell Signaling, USA) and rabbit polyclonal anti-β-actin antibodies (dilution 1 : 20,000; Santa Cruz Biotechnology, USA; sc-1616). Secondary anti-rabbit IgG (Bio-Rad) were used at 1 : 1000 and 1 : 10,000 dilutions for Bcl-xL and β-actin staining, respectively. The blots were developed with a SuperSignal™ West Femto Maximum Sensitivity Substrate chemiluminescence kit (Life Technologies, USA) and quantified after scanning with a ChemidocTM Touch Imaging System (Bio-Rad) using the Scion Image 4.0.3.2 program (Scion Corporation, USA). The amounts of Bcl-xL protein were expressed in arbitrary units relatively to the amounts of β-actin in the same sample.
The results on the levels of mRNAs and the corresponding proteins are presented as means ± SEM. The differences between the groups were estimated by one-way ANOVA with post-hoc comparison using Fisher’s LSD test. The differences were considered significant at p < 0.05.
RESULTS
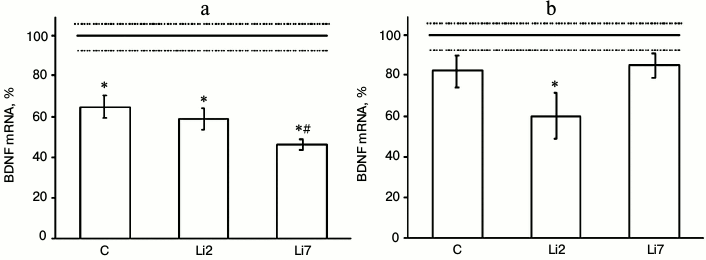
We found that in animals injected with physiological saline and stressed by repeated forced swimming showed considerable (almost 40%) decreased amount of BDNF mRNA in the hippocampus (Fig. 1a), but did not change the levels of this transcript in the frontal cortex (Fig. 1b) compared to non-stressed control animals. In rats injected with lithium for 2 days, the levels of BDNF mRNA in the hippocampus (Fig. 1a) and frontal cortex (Fig. 1b) were similar to those in the stressed rats receiving saline injections. However, in the frontal cortex, lithium exacerbated the suppressive effect of stress on BDNF expression and decreased BDNF mRNA content to a level that was significantly different from that in the non-stressed rats. In rats injected with lithium for 7 consecutive days, the levels of BDNF mRNA in the hippocampus significantly decreased compared to the control (rats injected with saline). The observed changes in BDNF expression suggest that both stress and lithium administration might modulate brain neural plasticity.
Fig. 1. Levels of BDNF mRNA in the hippocampus (a) and frontal cortex (b) of adult male rats after forced swimming-induced stress (expressed as a percentage of the mRNA levels in control). BDNF mRNA level in non-stressed animals (control) was taken as 100% and shown in the graph as a solid line; broken lines, ± SEM. C, rats injected with physiological saline; Li2 and Li7, rats injected with LiCl for 2 and 7 consecutive days, respectively; * p < 0.05, compared to non-stressed rats; # p < 0.05, compared to saline-treated rats.
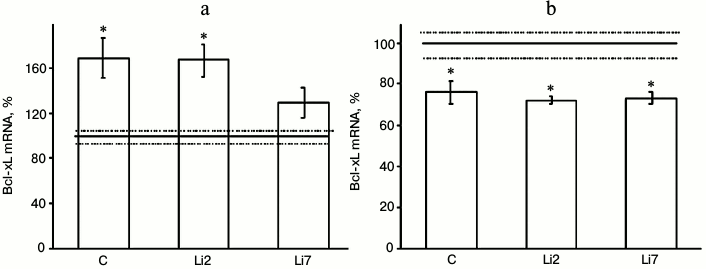
Both these factors also noticeably affected expression of the antiapoptotic protein Bcl-xL in the studied brain regions (Fig. 2). However, the effects of stress were much more pronounced than those of lithium. Thus, the levels of Bcl-xL mRNA increased more than 1.5-fold in the hippocampus (Fig. 2a) and decreased by one-third in the frontal cortex in the stressed animals compared to the non-stressed controls (Fig. 2b). At the same time, no changes in the Bcl-xL mRNA content were observed in the cortex after either 2- or 7-day administration of lithium. In the hippocampus, lithium injections for 2 days did not affect the stress-induced increase in the mRNA levels. When injected for 7 days, lithium decreased the levels of Bcl-xL mRNA in the stressed animals to the levels observed in the non-stressed controls.
Fig. 2. Levels of Bcl-xL mRNA in the hippocampus (a) and frontal cortex (b) of adult male rats after forced swimming-induced stress (expressed as a percentage of the mRNA levels in control). Bcl-xL mRNA level in non-stressed animals (control) was taken as 100% and is shown in the graph as a solid line; broken lines, ± SEM. C, rats injected with physiological saline; Li2 and Li7, rats injected with LiCl for 2 and 7 consequent days, respectively; * p < 0.05, compared to non-stressed rats.
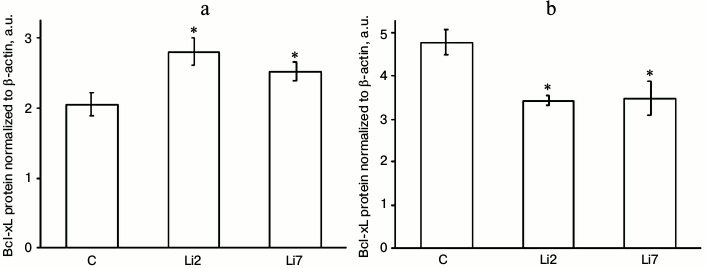
The effects of lithium on the levels of Bcl-xL protein in stressed animals were even more pronounced and were different in the studied brain regions (Fig. 3). Thus, in the hippocampus, lithium injections for either 2 or 7 days increased the content of Bcl-xL protein almost 1.5-fold (Fig. 3a), while in the cortex the same injections decreased the levels of Bcl-xL protein by a third (Fig. 3b) compared to the control animals injected with saline; i.e. the effects of lithium that were almost unnoticeable for the mRNA levels were more distinct for the Bcl-xL protein levels. Note the unidirectionality of the stress- and neuroleptic-induced changes in the mRNA transcript levels – an increase in the hippocampus and decrease in the frontal cortex.
Fig. 3. Levels of Bcl-xL protein (normalized to β-actin) in the hippocampus (a) and frontal cortex (b) of adult male rats after forced swimming-induced stress. C, rats injected with physiological saline; Li2 and Li7, rats injected with LiCl for 2 and 7 consequent days, respectively; * p < 0.05 compared to saline-treated rats.
Together, our results show that, similarly to many proteins involved in neural plasticity, expression of the antiapoptotic protein Bcl-xL in the brain can be modulated by stress and neuroleptics. The effects of these two factors depend on the brain region – activation of expression in the hippocampus and its suppression in the frontal cortex.
DISCUSSION
In this work, we demonstrated that the psychotropic agent lithium chloride can modulate expression of Bcl-xL, an antiapoptotic protein that has several properties typical for proteins involved in neural plasticity, in the frontal cortex and hippocampus of rats subjected to stress. The effects of lithium chloride were brain region-specific. Thus, stress and lithium administration activated Bcl-xL expression in the hippocampus, but suppressed it (both the mRNA transcript and the Bcl-xL protein) in the frontal cortex. Note that there was no clear correlation between the levels of the mRNA transcript and the amounts of synthesized Bcl-xL protein.
Since the effects of lithium on Bcl-xL expression in vivo were observed for the first time, there are no data so far that would allow discussion of possible mechanisms of action of lithium on the expression of the bcl-xL gene. A similar effect of lithium has been described for BDNF, whose involvement into neural plasticity in the brain has been well studied. LiCl was found to decrease BDNF protein levels in the cortex, but not in the hippocampus [16]. At the same time, activation of BDNF TrkB receptors by a long-term lithium administration was observed in the cortex, but not in the hippocampus [17]. Similar region-specific effects of lithium were also found for proteins of other signaling systems. For example, lithium significantly changed expression of subunits of guanidine-binding protein in the hippocampus, but produced no similar effect in the cortex [18].
The observed stress- and lithium-induced changes in the BDNF mRNA levels indicate that both these factors can modulate brain neural plasticity. These changes represent some of the numerous effects of lithium on BDNF gene expression [14, 15]. It was demonstrated earlier that lithium increased the content of BDNF in a cell culture [19]. In addition, BDNF levels in the hippocampus and the cortex increased after 2- to 4-week (but not 1-week) administration of lithium [20]. However, there are also contradicting data that lithium produced no effect on the contents of BDNF protein [21] and mRNA [22] or that it even decreases BDNF transcript levels [23, 24]. The discrepancies between the effects of lithium on the mRNA and protein levels observed in our work are not unique for Bcl-xL and have been described in several studies [21, 23] for BDNF mRNA and protein levels in nerve tissue.
The effects of lithium on the expression of the antiapoptotic Bcl-2 protein [14, 15] have also been studied. Thus, incubation of cerebellum granular cell culture in the presence of LiCl for 7 days increased the levels of both Bcl-2 mRNA and protein in the cells [25]. Activation of Bcl-2 expression by lithium has also been demonstrated in vivo [26]. Recently, lithium was found to increase the levels of mRNAs for Bcl-2 and Bcl-xL in hippocampal neuron culture [19]. The in vivo data obtained in our work correlate well with the in vitro results and suggest that lithium might have some neuroprotective effect mediated by Bcl-xL, at least in the hippocampus. Out of 62 genes that are expressed differently in bipolar depression patients in response to lithium treatment, the most pronounced difference in the levels of gene expression was observed for Bcl-xL (BCL2L1) [27]. According to the authors, increased Bcl-xL expression in the blood cells of patients responding to lithium treatment (but not in nonresponding or untreated individuals) indicates a specific role of this gene in the therapeutic response to the neuroleptic. The results of our work show that lithium increases Bcl-xL expression not only in blood cells [27] or in a cell culture [19], but also in the rat hippocampus, which is the most important brain structure involved in the regulation of the organism’s psychosomatic status.
In conclusion, we showed that Bcl-xL expression in the brain is affected by stress and the action of neuroleptics in a region-specific manner – Bcl-xL expression is activated in the hippocampus and suppressed in the frontal cortex. Activation of Bcl-xL expression in the hippocampus by lithium, which was observed in this work for the first time, suggests that this antiapoptotic protein might mediate the therapeutic effects of the neuroleptic in the treatment of psychoemotional disorders.
Acknowledgements
This work was supported by the State Task Budget (project No. 0324-2015-0004) and by the Russian Foundation for Basic Research (project No. 14-04-00132).
REFERENCES
1.Lucassen, P. J., Meerlo, P., Naylor, A. S., Van
Dam, A. M., Dayer, A. G., Fuchs, E., Oomen, C. A., and Czeh, B. (2010)
Regulation of adult neurogenesis by stress, sleep disruption, exercise
and inflammation: implications for depression and antidepressant
action, Eur. Neuropsychopharmacol., 20, 1-17.
2.Chao, C. C., Ma, Y. L., and Lee, E. H. (2011)
Brain-derived neurotrophic factor enhances Bcl-xL expression through
protein kinase casein kinase 2-activated and nuclear factor kappa
B-mediated pathway in rat hippocampus, Brain Pathol., 21,
150-156.
3.Jonas, E. (2006) Bcl-xL regulates synaptic
plasticity, Mol. Interv., 6, 208-222.
4.Gal, A., Pentelenyi, K., Remenyi, V., Wappler, E.
A., Safrany, G., Skopal, J., and Nagy, Z. (2009) Bcl-2 or Bcl-xL gene
therapy increases neural plasticity proteins nestin and c-fos
expression in PC12 cells, Neurochem. Int., 55,
349-353.
5.Li, H., Chen, Y., Jones, A. F., Sanger, R. H.,
Collis, L. P., Flannery, R., McNay, E. C., Yu, T., Schwarzenbacher, R.,
Bossy, B., Bossy-Wetzel, E., Bennett, M. V., Pypaert, M., Hickman, J.
A., Smith, P. J., Hardwick, J. M., and Jonas, E. A. (2008) Bcl-xL
induces Drp1-dependent synapse formation in cultured hippocampal
neurons, Proc. Natl. Acad. Sci. USA, 105, 2169-2174.
6.Li, H., Alavian, K. N., Lazrove, E., Mehta, N.,
Jones, A., Zhang, P., Licznerski, P., Graham, M., Uo, T., Guo, J.,
Rahner, C., Duman, R. S., Morrison, R. S., and Jonas, E. A. (2013) A
Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during
endocytosis, Nat. Cell. Biol., 15, 773-785.
7.Lanshakov, D. A., Drozd, U. S., and Dygalo, N. N.
(2017) Optogenetic stimulation increases level of anti-apoptotic Bcl-xL
protein in neurons, Biochemistry (Moscow), 82,
340-344.
8.Shishkina, G. T., Kalinina, T. S., Berezova, I. V.,
Bulygina, V. V., and Dygalo, N. N. (2010) Resistance to the development
of stress-induced behavioral despair in the forced swim test associated
with elevated hippocampal Bcl-xl expression, Behav. Brain Res.,
213, 218-224.
9.Dygalo, N. N., Kalinina, T. S., Bulygina, V. V.,
and Shishkina, G. T. (2012) Increased expression of the anti-apoptotic
protein Bcl-xL in the brain is associated with resilience to
stress-induced depression-like behavior, Cell. Mol. Neurobiol.,
32, 767-776.
10.Shishkina, G. T., Kalinina, T. S., Berezova, I.
V., and Dygalo, N. N. (2012) Stress-induced activation of the brainstem
Bcl-xL gene expression in rats treated with fluoxetine: correlations
with serotonin metabolism and depressive-like behavior,
Neuropharmacology, 62, 177-183.
11.Shishkina, G. T., Kalinina, T. S., Bulygina, V.
V., Lanshakov, D. A., Babluk, E. V., and Dygalo, N. N. (2015)
Anti-apoptotic protein Bcl-xL expression in the midbrain raphe region
is sensitive to stress and glucocorticoids, PLoS One, 10,
e0143978.
12.Bauer, M. S., and Mitchner, L. (2004) What is a
“mood stabilizer”? An evidence-based response, Am. J.
Psychiatry, 161, 3-18.
13.Dell’Osso, L., Del Grande, C., Gesi, C.,
Carmassi, C., and Musetti, L. (2016) A new look at an old drug:
neuroprotective effects and therapeutic potentials of lithium salts,
Neuropsychiatr. Dis. Treat., 11, 1687-1703.
14.Hammonds, M. D., and Shim, S. S. (2009) Effects
of 4-week treatment with lithium and olanzapine on levels of
brain-derived neurotrophic factor, B-cell CLL/lymphoma 2 and
phosphorylated cyclic adenosine monophosphate response element-binding
protein in the sub-regions of the hippocampus, Basic Clin.
Pharmacol. Toxicol., 105, 113-119.
15.Quiroz, J. A., Machado-Vieira, R., Zarate, C. A.,
Jr., and Manji, H. K. (2010) Novel insights into lithium’s
mechanism of action: neurotrophic and neuroprotective effects,
Neuropsychobiology, 62, 50-60.
16.Angelucci, F., Aloe, L., Jimenez-Vasquez, P., and
Mathe, A. A. (2003) Lithium treatment alters brain concentrations of
nerve growth factor, brain-derived neurotrophic factor and glial cell
line-derived neurotrophic factor in a rat model of depression, Int.
J. Neuropsychopharmacol., 6, 225-231.
17.Rantamaki, T., Knuuttila, J. E., Hokkanen, M. E.,
and Castren, E. (2006) The effects of acute and long-term lithium
treatments on TrkB neurotrophin receptor activation in the mouse
hippocampus and anterior cingulate cortex, Neuropharmacology,
50, 421-277.
18.McGowan, S., Eastwood, S. L., Mead, A., Burnet,
P. W., Smith, C., Flanigan, T. P., and Harrison, P. J. (1996)
Hippocampal and cortical G protein (Gs alpha, G(o) alpha and Gi2 alpha)
mRNA expression after electroconvulsive shock or lithium carbonate
treatment, Eur. J. Pharmacol., 306, 249-255.
19.Dwivedi, T., and Zhang, H. (2015) Lithium-induced
neuroprotection is associated with epigenetic modification of specific
BDNF gene promoter and altered expression of apoptotic-regulatory
proteins, Front. Neurosci., 14, 457.
20.Fukumoto, T., Morinobu, S., Okamoto, Y., Kagaya,
A., and Yamawaki, S. (2001) Chronic lithium treatment increases the
expression of brain-derived neurotrophic factor in the rat brain,
Psychopharmacology (Berlin), 158, 100-106.
21.Emamghoreishi, M., Keshavarz, M., and Nekooeian,
A. A. (2015) Acute and chronic effects of lithium on BDNF and GDNF mRNA
and protein levels in rat primary neuronal, astroglial and
neuroastroglia cultures, Iran J. Basic. Med. Sci., 18,
240-246.
22.Stertz, L., Fries, G. R., Aguiar, B. W.,
Pfaffenseller, B., Valvassori, S. S., Gubert, C., Ferreira, C. L.,
Moretti, M., Cereser, K. M., and Kauer-Sant’Anna, M. (2014)
Histone deacetylase activity and brain-derived neurotrophic factor
(BDNF) levels in a pharmacological model of mania, Rev. Bras.
Psiquiatr., 36, 39-46.
23.Jacobsen, J. P., and Mork, A. (2004) The effect
of escitalopram, desipramine, electroconvulsive seizures and lithium on
brain-derived neurotrophic factor mRNA and protein expression in the
rat brain and the correlation to 5-HT and 5-HIAA levels, Brain
Res., 1024, 183-192.
24.Hanson, N. D., Nemeroff, C. B., and Owens, M. J.
(2011) Lithium, but not fluoxetine or the corticotropin-releasing
factor receptor 1 receptor antagonist R121919, increases cell
proliferation in the adult dentate gyrus, J. Pharmacol. Exp.
Ther., 337, 180-186.
25.Chen, R. W., and Chuang, D. M. (1999) Long-term
lithium treatment suppresses p53 and Bax expression but increases Bcl-2
expression. A prominent role in neuroprotection against excitotoxicity,
J. Biol. Chem., 274, 6039-6042.
26.Manji, H. K., Moore, G. J., and Chen, G. (2000)
Clinical and preclinical evidence for the neurotrophic effects of mood
stabilizers: implications for the pathophysiology and treatment of
manic-depressive illness, Biol. Psychiatry, 48,
740-754.
27.Beech, R. D., Leffert, J. J., Lin, A., Sylvia, L.
G., Umlauf, S., Mane, S., Zhao, H., Bowden, C., Calabrese, J. R.,
Friedman, E. S., Ketter, T. A., Iosifescu, D. V., Reilly-Harrington, N.
A., Ostacher, M., Thase, M. E., and Nierenberg, A. (2014)
Gene-expression differences in peripheral blood between lithium
responders and non-responders in the Lithium Treatment-Moderate Dose
Use Study (LiTMUS), Pharmacogenom. J., 14, 182-191.