Cooperative Synthesis of Dopamine in Rat Mediobasal Hypothalamus as a Compensatory Mechanism in Hyperprolactinemia
A. U. Kurina*, T. S. Pronina, L. K. Dilmukhametova, G. V. Maleev, and M. V. Ugrumov
Koltzov Institute of Developmental Biology, Russian Academy of Sciences, 119334 Moscow, Russia; E-mail: kurina.anya@gmail.com* To whom correspondence should be addressed.
Received October 18, 2016; Revision received November 29, 2016
Dopamine (DA), synthesized in the mediobasal hypothalamus by dopaminergic neurons containing two enzymes of DA synthesis – tyrosine hydroxylase and decarboxylase of aromatic L-amino acids, or by monoenzymatic non-dopaminergic neurons containing one DA synthesis enzyme in cooperation, is known to have an inhibitory effect on prolactin secretion. Deterioration of this inhibitory control leads to an increase in prolactin concentration in the blood and to the development of hyperprolactinemia syndrome. In a rat model of hyperprolactinemia induced by administration of a neurotoxin causing degeneration of dopaminergic and noradrenergic neurons, the level of DA first decreases, leading to an increase in prolactin level (decompensation stage), while later both levels are restored to normal (compensation stage). However, the mechanism of such compensation is still not clear. The aim of the present study was to analyze whether the increase in cooperative synthesis of DA by monoenzymatic neurons during hyperprolactinemia is a manifestation of a compensatory mechanism representing a particular case of neuroplasticity. The level of cooperative synthesis in the hyperprolactinemia model and in the control was estimated as the level of synthesis of DA and L-dihydroxyphenylalanine (L-DOPA) – an intermediate product of DA synthesis, when L-DOPA transfer from neurons containing tyrosine hydroxylase into neurons containing aromatic L-amino acid decarboxylase is inhibited. The level of DA synthesis during the decompensation stage was not changed, while during the compensation stage it was lower than the control. Along with a reduction in DA level, during the compensation stage an increase in the extracellular L-DOPA level in the medium was detected. Thus, the compensation of DA deficiency after degeneration of dopaminergic neurons in the mediobasal hypothalamus is due to the increase in cooperative synthesis of DA by monoenzymatic neurons containing one of the complementary enzymes of the DA synthesis pathway.
KEY WORDS: mediobasal hypothalamus, neuroplasticity, dopamine, noradrenaline, L-dihydroxyphenylalanine, 6-hydroxydopamine, high-performance liquid chromatographyDOI: 10.1134/S0006297917030154
Abbreviations: AADC, aromatic L-amino acid decarboxylase; DA, dopamine; DA-ergic, dopaminergic; 6-OHDA, 6-hydroxydopamine; L-DOPA, L-dihydroxyphenylalanine; MBH, mediobasal hypothalamus; TH, tyrosine hydroxylase.
Dopamine (DA), synthesized in neurons of the mediobasal hypothalamus
(MBH) and released in the median eminence to the pituitary portal
system, has an inhibitory effect on prolactin secretion by pituitary
lactotrophs [1]. Degeneration of dopaminergic
(DA-ergic) neurons of the MBH is accompanied by hyperprolactinemia
syndrome, characterized by an increase in prolactin level in blood,
leading to the impairment of the reproductive function of the organism.
DA-ergic neurons possess two enzymes in the DA synthesis
pathway – tyrosine hydroxylase (TH), catalyzing the
transformation of L-tyrosine into L-dihydroxyphenylalanine (L-DOPA),
and aromatic L-amino acid decarboxylase (AADC), catalyzing the
transformation of L-DOPA into DA [2-4]. Earlier, we obtained direct evidence that DA is
synthesized not only by DA-ergic neurons, but also by monoenzymatic
neurons containing only one of the two DA synthesis
enzymes – TH or AADC. The intermediate product of DA
synthesis – L-DOPA – is secreted into the
intercellular space by TH-expressing neurons and is delivered by the
neutral amino acid transporter into AADC-expressing neurons, where DA
is finally synthesized [5, 6].
To study cellular and molecular mechanisms of the development of hyperprolactinemia, experimental models based on intraventricular injection of a neurotoxin – 6-hydroxydopamine (6-OHDA) that causes the degeneration of catecholaminergic (DA-ergic and noradrenergic) neurons is used [7, 8]. Using this model, we showed earlier that on the 14th day after injection of 6-OHDA, a decrease in DA level in MBH and an increase in prolactin level in blood are observed, i.e. decompensation is taking place. However, on the 45th day both parameters are restored to the normal level [9], i.e. compensation is observed. We propose a hypothesis that such compensation is assured by mechanisms of neuroplasticity, in particular by an increase in cooperative DA synthesis by monoenzymatic neurons.
Thus, the aim of the present work was to test whether the increase in cooperative synthesis of dopamine by monoenzymatic neurons during hyperprolactinemia in rats is a result of a compensatory mechanism, being a particular case of neuroplasticity. To achieve this goal, we checked: (i) L-DOPA synthesis in TH-expressing neurons, and (ii) contribution of monoenzymatic neurons to DA synthesis on the 14th day (decompensation stage) and on the 45th day (compensation stage) after injection of 6-OHDA (with corresponding controls) when the uptake of L-DOPA by AADC-expressing neurons is inhibited.
MATERIALS AND METHODS
Animals. Experiments were done with 40 male Wistar rats weighing 220-250 g. To animals of the experimental group, 250 µg of 6-OHDA (Sigma, USA) dissolved in 15 µl of 0.9% NaCl (Sigma) with 0.1% ascorbic acid (Sigma) was injected into the lateral ventricle stereotaxically (coordinates from bregma: caudally – 0.8 mm, laterally – 1.5 mm, 3.2 mm from the brain surface) [10]; to animals of the control group, 0.9% NaCl with 0.1% ascorbic acid were injected, with a rate of 3 µl/min in both cases. The rats were kept under standard vivarium conditions with free access to food and water and 12-h day/night cycle. All manipulations with animals were carried out according to the national and international ethics guidelines and were approved by the Bioethics Commission of the Koltzov Institute of Developmental Biology, Russian Academy of Sciences.
Perfusion of MBH slices. On the 14th or 45th day after injection of the neurotoxin or 0.9% NaCl (n = 10 for each group), the rats were decapitated under anesthetic (chloral hydrate 400 mg/kg; Sigma) and the brain was removed. Five 250-µm thick brain slices with coordinates from 1.92 to 3.36 mm caudally from the bregma were cut using a Vibratome 1000 plus (Sectioning System, Germany) in Krebs–Ringer solution (120 mM NaCl, 4.8 mM KCl, 2 mM CaCl2, 1.3 mM MgSO4, 25 mM NaHCO3, 10 mM D-glucose, 20 mM HEPES, 0.1 mM ascorbic acid, pH 7.4) (Sigma) at 4°C. The MBH was dissected out of these vibratome slices using a binocular magnifier, divided in two symmetrical halves – left and right (Fig. 1) – and placed into a 400-µl thermostat chamber (37°C) and perfused with Krebs–Ringer solution. Constant 100 µl/min flow rate was provided by a peristaltic pump (Ismatec, Germany). During stabilization of the system for the first 40 min, samples were not collected; then, slices from the right half of the MBH were incubated in Krebs–Ringer solution, while slices from the left half – in Krebs–Ringer solution supplemented with 0.5 mM L-leucine (Sigma). Three consecutive 20-min fractions of the incubation medium flowing from the chambers were collected. L-leucine was used to inhibit the uptake of L-DOPA by monoenzymatic AADC-expressing neurons, since it competes with L-DOPA for binding with the neutral amino acid transporter. Collected fractions and slices after incubation were frozen in liquid nitrogen and stored at –70°C until concentrations of DA and L-DOPA were analyzed.
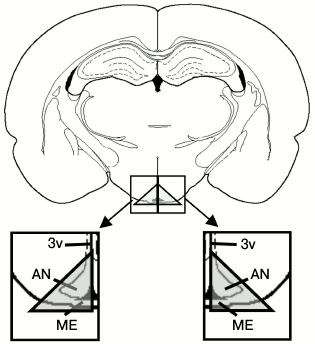
Fig. 1. Scheme of the isolated area of mediobasal hypothalamus from vibratome brain slices (2.3 mm from bregma). AN, arcuate nucleus; ME, median eminence; 3v, third ventricle.
High-performance liquid chromatography with electrochemical detection (HPLC-EC). Concentrations of DA and L-DOPA in collected fractions of incubation medium and in MBH slices were measured using HPLC-EC. The tissue was homogenized with an ultrasonic homogenizer (Labsonic M; Sartorius, France) in 110 µl of 0.1 M HClO4 (Sigma) containing 1 ng of 3,4-dihydroxybenzylamine as an internal standard. The homogenates were centrifuged for 10 min at 15,000g at 4°C, and the supernatant was collected. Then 10 mg of aluminum oxide (Sigma) was added to each fraction of the incubation media (1 ml), the samples were incubated for 15 min at 4°C with shaking, and then they were centrifuged for 10 min at 1200g at 4°C. The pellets were supplemented with 100 µl of 0.2 M HClO4, the samples were again centrifuged for 10 min at 1200g, and the supernatant was collected. Monoamines were separated using a 10-cm reverse-phase column with 3-mm internal diameter filled with Reprosil-Pur C18.5 µm (Elsico, Russia) at voltage 850 mV. The mobile phase consisted of 0.1 M citrate-phosphate buffer containing 0.3 mM sodium octanesulfonate, 0.1 mM EDTA, and 12% acetonitrile (pH 3.0) (Sigma). A flow rate 800 µl/min was provided by a peristaltic pump (Gilson, France). The DA and L-DOPA peaks on the chromatogram were identified in accordance with their elution times in the standard solution. The chromatograms were registered and analyzed using MultiChrom v.1.5 software (Ampersand Ltd., USA). DA and L-DOPA concentrations were estimated using the internal standard technique, analyzing the ratio of the peak areas of the internal standard and sample using the MultiChrom software (Russia).
Statistics. Normal distribution within each group was analyzed using the Shapiro–Wilk and Kolmogorov–Smirnov criteria with integrated programs GraphPad Prism v.6.0 for Windows (GraphPad Software, USA). Student’s t-test was used for statistical analysis of collected data. Standard error of mean was calculated at the level of probability 95% (p < 0.05).
RESULTS
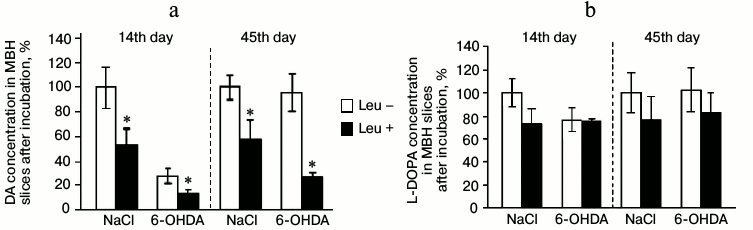
Fourteenth day after stereotactic injection of 6-OHDA to experimental animals or 0.9% NaCl to control animals. Concentrations of DA and L-DOPA in MBH slices after incubation. On the 14th day after injection of 6-OHDA (experimental group), the concentration of DA in MBH slices after incubation in Krebs–Ringer solution containing L-leucine decreased by 48% compared to that after incubation in Krebs–Ringer solution without addition of L-leucine. After injection of 0.9% of NaCl (control group), the concentration of DA in MBH slices after incubation in Krebs–Ringer solution containing L-leucine was 47% lower compared to that after incubation in Krebs–Ringer solution without L-leucine (Fig. 2a).
Fig. 2. Concentration of (a) DA and (b) L-DOPA in slices of mediobasal hypothalamus after incubation in Krebs–Ringer solution with addition of L-leucine (Leu+) or without (Leu–) on the 14th and 45th day after stereotactic injection of 0.9% NaCl in the control group and 6-OHDA in the experimental group (n = 10 for each group); * p < 0.05 (Leu+ compared to Leu–).
On the 14th day after injection of 6-OHDA, the concentration of L-DOPA in MBH slices incubated in Krebs–Ringer solution with addition of L-leucine did not change compared to L-DOPA concentration in MBH slices incubated in Krebs–Ringer solution without L-leucine. The concentration of L-DOPA in MBH slices of the control group incubated in Krebs–Ringer solution with addition of L-leucine also did not change compared to the concentration of L-DOPA after incubation without L-leucine (Fig. 2b).
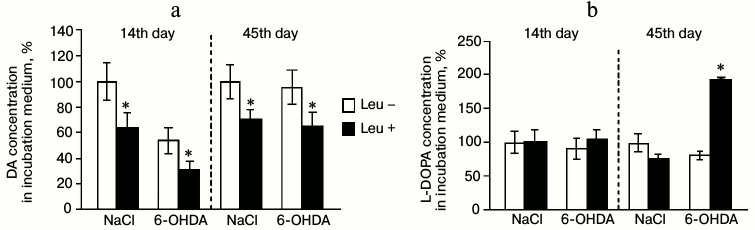
Concentrations of DA and L-DOPA in the perfusion medium. On the 14th day after injection of 6-OHDA, the concentration of DA in the perfusion medium fractions decreased by 33% after incubation in Krebs–Ringer solution with addition of L-leucine compared to DA concentration after incubation in Krebs–Ringer solution without L-leucine. Addition of L-leucine to the perfusion medium of the control group led to 37% decrease in DA concentration in the perfusion medium fractions compared to DA concentration after incubation in Krebs–Ringer solution without L-leucine (Fig. 3a).
Fig. 3. Concentration of (a) DA and (b) L-DOPA in the medium after incubation of mediobasal hypothalamus slices in Krebs–Ringer solution with addition of L-leucine (Leu+) or without (Leu–) on the 14th and 45th day after stereotactic injection of 0.9% NaCl in the control and 6-OHDA in the experiment (n = 10 for each group); * p < 0.05 (Leu+ compared to Leu–).
Incubation of MBH slices with L-leucine had no effect on L-DOPA concentration in the perfusion medium fractions compared to slices incubated without L-leucine in the experimental and in the control groups (Fig. 3b).
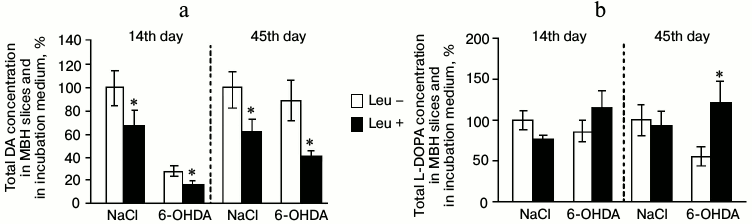
Total concentrations of DA and L-DOPA in MBH slices and in the perfusion medium. After perfusion of MBH slices in Krebs–Ringer solution with addition of L-leucine, total DA concentration decreased by 37% in the experimental group and by 41% in the control group compared to samples incubated in Krebs–Ringer solution without addition of L-leucine (Fig. 4a).
Fig. 4. Total concentration of (a) DA and (b) L-DOPA in mediobasal hypothalamus slices and in the medium after incubation in Krebs–Ringer solution with addition of L-leucine (Leu+) or without (Leu–) on the 14th and 45th day after stereotactic injection of 0.9% NaCl in the control group and 6-OHDA in the experimental group (n = 10 for each group); * p < 0.05 (Leu+ compared to Leu–).
On the 14th day after stereotactic injection, both in the experimental and in the control groups total the concentration of L-DOPA in slices and in the perfusion medium after incubation in Krebs–Ringer solution with addition of L-leucine was not significantly changed compared to the total concentration of L-DOPA after incubations in Krebs–Ringer solution without L-leucine (Fig. 4b).
Forty-fifth day after stereotactic injection of 6-OHDA to experimental animals or 0.9% NaCl to control animals. Concentrations of DA and L-DOPA in MBH slices after incubation. On the 45th day after injection of 6-OHDA, the concentration of DA in MBH slices after incubation in Krebs–Ringer solution containing L-leucine decreased by 72% compared to the DA concentration in slices incubated in Krebs–Ringer solution without addition of L-leucine. The concentration of DA in MBH slices of the control group after incubation in Krebs–Ringer solution with addition of L-leucine decreased by 34% compared to DA concentration after incubation without L-leucine (Fig. 2a).
In the experimental and the control groups, the concentration of L-DOPA in MBH slices after incubation in Krebs–Ringer solution with addition of L-leucine did not change compared to the incubation in Krebs–Ringer solution without addition of L-leucine (Fig. 2b).
Concentrations of DA and L-DOPA in the perfusion medium. After perfusion of MBH slices in Krebs–Ringer solution with addition of L-leucine, the DA concentration in fractions of the perfusion medium decreased by 30% for the experimental group and by 29% for the control group compared to samples incubated in Krebs–Ringer solution without addition of L-leucine (Fig. 3a).
On the 45th day after injection of 6-OHDA, the concentration of L-DOPA in fractions of the perfusion medium increased by 93% after incubation in Krebs–Ringer solution with addition of L-leucine, compared to L-DOPA concentration after incubation in Krebs–Ringer solution without addition of L-leucine. The concentration of L-DOPA in fractions of the perfusion medium of the control group after incubation in Krebs–Ringer solution with addition of L-leucine was not changed compared to the concentration of L-DOPA in fractions of the perfusion medium after incubation in Krebs–Ringer solution without addition of L-leucine (Fig. 3b).
Total concentrations of DA and L-DOPA in MBH slices and in the perfusion medium. After perfusion of MBH slices in Krebs–Ringer solution with addition of L-leucine, the total concentration of DA in slices and in the perfusion medium decreased by 55% in the experimental group and by 36% in the control group compared with total concentration after incubation in Krebs–Ringer solution without addition of L-leucine (Fig. 4a).
In the experimental group, total L-DOPA concentration in MBH slices and in the perfusion medium after incubation in Krebs–Ringer solution with addition of L-leucine increased by 65% compared to the total L-DOPA concentration after incubation in Krebs–Ringer solution without addition of L-leucine. In the control group, incubation in Krebs–Ringer solution containing L-leucine did not change the total L-DOPA concentration compared to that after incubation without L-leucine (Fig. 4b).
DISCUSSION
It was shown earlier that medialization of hyperprolactinemia by administration of 6-OHDA starts with DA deficit in the MBH and the increase of prolactin level in blood, but later both parameters return to normal levels [7, 9]. Taking this into account, in the present study we analyzed samples on the 14th day after toxin administration, i.e. at the decompensation stage, when the degeneration of neurons induced by the toxin should be accomplished, and on the 45th day, i.e. at the compensation stage, when compensatory processes – brain plasticity mechanisms – have occurred. Degeneration of DA-ergic neurons and the consequent DA deficiency can trigger several compensatory processes, such as: (i) enhancement of DA synthesis by the remaining DA-ergic neurons; (ii) activation of expression of genes coding for DA synthesis enzymes in non-DA-ergic neurons and initiation of cooperative synthesis of DA by TH-expressing and AADC-expressing neurons; (iii) projection of axons of DA-ergic neurons from brain areas not damaged by the toxin [5, 11].
The aim of this work was to test the hypothesis that the increase in cooperative synthesis of DA by monoenzymatic neurons in MBH is the mechanism of hyperprolactinemia compensation in rats.
Cooperative synthesis was analyzed according to our original methodology described earlier [6], when MBH slices were incubated in Krebs–Ringers solution containing competitive inhibitor of L-DOPA uptake by AADC-containing neurons, thus preventing cooperative synthesis of DA by monoenzymatic neurons. Incubation of MBH vibratome slices allows measuring the release of DA and L-DOPA into the medium and their accumulation in the tissue during several hours in vitro. Unlike the original methodology, in this work only perfusion was used, which, contrary to static incubation, excludes the influence of synthesis and degradation products accumulated in the incubation medium on the metabolism of catecholamines due to the rapid removal of the released mediator with the flow of the perfusion medium [12].
L-DOPA uptake from the intercellular space to the AADC-containing neurons is performed by a relatively specific membrane transporter, which also transfers large neutral amino acids such as L-leucine, L-tyrosine, etc. Because all these amino acids compete for the same membrane carrier [13-15], they can be used as competitive inhibitors of L-DOPA uptake by monoenzymatic AADC-containing neurons. Using MBH slices of normal animals, we showed earlier that L-leucine inhibits L-DOPA uptake from the intercellular space by monoenzymatic AADC-expressing neurons, thus blocking the cooperative synthesis of DA by monoenzymatic neurons [5, 6]. In the present study, L-leucine was used also because it is not a precursor of L-DOPA and, therefore it has no effect on DA metabolism.
The effect of L-leucine as an inhibitor of L-DOPA uptake by AADC-containing neurons on synthesis of DA and L-DOPA was estimated by comparison of DA and L-DOPA concentration in the perfusion medium and in MBH slices after incubation in Krebs–Ringer solution with or without addition of L-leucine. As an index of DA synthesis level, the total concentration of DA or L-DOPA was estimated as the sum of their concentrations in slices after incubation and in the perfusion medium. A decrease in DA concentration in MBH slices and in the perfusion medium in the presence of L-leucine blocking the uptake of L-DOPA by monoenzymatic neurons should be considered as a quantitative index of DA synthesis by monoenzymatic TH-containing neurons in cooperation with AADC-expressing neurons [5].
Using this methodology, we have shown here that during the decompensation stage on the 14th day after injection of 6-OHDA the level of DA synthesis in the presence of L-leucine did not change compared to the control group. In other words, addition of L-leucine to the perfusion medium led to similar decrease in DA concentration in slices after incubation and in the perfusion medium in the control and experimental groups compared to samples incubated without L-leucine. During the compensation stage on the 45th day in a similar experiment, the level of DA synthesis in the presence of L-leucine decreased significantly compared to the control group and compared to the experimental group on the 14th day, which indicates the increase in cooperative synthesis of DA by monoenzymatic neurons. DA concentration in slices is the result of dopamine synthesis and accumulation, while DA concentration in the perfusion medium is the result of a spontaneous release of dopamine [16]. Considerable decrease in DA concentration on the 45th day after injection of 6-OHDA was observed only in MBH slices after incubation with L-leucine, which indicates the decrease in DA synthesis after blocking of its cooperative synthesis by monoenzymatic neurons. Enhancement of cooperative synthesis of DA on the 45th day after injection of 6-OHDA agrees with our previous results concerning the restoration of the DA level [9].
In addition to DA concentration, the concentration of L-DOPA in slices after incubation and in the perfusion medium was measured. The level of L-DOPA in slices after incubation did not change. This is probably because L-DOPA is synthesized in the cytoplasm and does not accumulate in the tissue. This hypothesis is based on the fact that in the bienzymatic dopaminergic neurons the next step of dopamine synthesis from L-DOPA catalyzed by the second enzyme of dopamine synthesis – AADC – also takes place in the cytoplasm [17]. Moreover, the level of L-DOPA synthesis in the incubation media in the presence of L-leucine increased considerably during the compensation stage on the 45th day after injection of 6-OHDA, which shows the activation of L-DOPA synthesis in TH-expressing neurons compared to the decompensation stage.
In MBH, TH-expressing neurons [18, 19] and AADC-expressing neurons [18, 20] were identified using the immunohistochemical method. Our data agrees with previous results showing that in MBH of rats, injection of 6-OHDA causes degeneration of about half of the DA-ergic neurons, while the number of monoenzymatic neurons increases [5]. In mammals, monoenzymatic neurons containing TH or AADC have been found in many other brain areas in adults. For example, in the striatum of rodents monoenzymatic neurons are revealed only in the case of neurodegeneration [21, 22], and in primates under normal conditions many TH-containing neurons are revealed [23-25], while in case of neurodegeneration their number increases dramatically [23, 24]. This shows a possible role of cooperative synthesis of DA by these neurons as a plasticity mechanism that is activated in case of functional insufficiency of DA-ergic neurons not only in MBH, but also in other brain regions.
In conclusion, we have shown that an increase in DA level in MBH observed in rats on the 45th day after administration of a neurotoxin is caused by enhancement of cooperative synthesis of dopamine by non-DA-ergic monoenzymatic neurons and is a manifestation of a compensatory mechanism as a particular case of neuroplasticity.
Acknowledgements
This study was supported by the Russian Science Foundation (project No. 14-15-01122).
REFERENCES
1.Ben-Jonathan, N., and Hnasko, R. (2001) Dopamine as
a prolactin (PRL) inhibitor, Endocr. Rev., 22,
724-763.
2.Bjorklund, A., and Lindvall, O. (1984)
Dopamine-containing systems in CNS, in Handbook of Chemical
Neuroanatomy (Bjorklund, A., and Hokfelt, T., eds.) Vol. 2,
Elsevier, Amsterdam, pp. 55-122.
3.Dahlström, A., and Fuxe, K. (1964) Evidence
for the existence of monoamine-containing neurons in the central
nervous system. I. Demonstration of monoamines in the cell bodies of
brain stem neurons, Acta Physiol. Scand., 232,
S1-S55.
4.Hoookfelt, T., Johansson, O., and Goldstein, M.
(1984) Central catecholamine neurons as revealed by
immunohistochemistry with special reference to adrenaline neurons, in
Handbook of Chemical Neuroanatomy (Bjorklund, A., and Hokfelt,
T., eds.) Vol. 2, Elsevier, Amsterdam, pp. 157-276.
5.Ugrumov, M. V. (2013) Brain neurons partly
expressing dopaminergic phenotype: location, development, functional
significance, and regulation, Adv. Pharmacol., 68,
37-91.
6.Ugrumov, M., Taxi, J., Pronina, T., Kurina, A.,
Sorokin, A., Sapronova, A., and Calas, A. (2014) Neurons expressing
individual enzymes of dopamine synthesis in the mediobasal hypothalamus
of adult rats: functional significance and topographic interrelations,
Neuroscience, 277, 45-54.
7.Fenske, M., and Wuttke, W. (1976) Effects of
intraventricular 6-hydroxydopamine injections on serum prolactin and LH
levels: absence of stress-induced pituitary prolactin release, Brain
Res., 104, 63-70.
8.Jonsson, G. (1983) Chemical lesioning techniques:
monoamine neurotoxins, in Handbook of Chemical Neuroanatomy
(Bjorklund, A., and Hokfelt, T., eds.) Vol. 1, Elsevier, Amsterdam, pp.
463-507.
9.Ziyazetdinova, G. Z., Sapronova, A. Ya., Kiyasov,
V. Ya., Nanaev, A. K., Kudrin, V. S., Martina, N., Tillier, I., and
Ugrumov, M. V. (2008) Compensatory reaction during degeneration of
arcuate nucleus dopaminergic neurons in rats, J. Evol. Biochem.
Physiol., 44, 82-88.
10.Paxinos, G., and Watson, C. (1997) The Rat
Brain in Stereotaxic Coordinates, 3rd Edn., Academic Press,
London.
11.Vos, P. E., Steinbusch, H. W., Ronken, E., and
Van Ree, J. M. (1999) Short and long term plasticity after lesioning of
the cell body or terminal field area of the dopaminergic
mesocorticolimbic system in the rat, Brain Res., 831,
237-247.
12.Mulder, A. H., Van Den Berg, W. B., and Stoof, J.
C. (1975) Calcium-dependent release of radiolabeled catecholamines and
serotonin from rat brain synaptosomes in a superfusion system, Brain
Res., 99, 419-424.
13.Misu, Y., Kitahama, K., and Goshima, Y. (2003)
L-3,4-dihydroxyphenylalanine as a neurotransmitter candidate in the
central nervous system, Pharmacol. Ther., 97,
117-137.
14.Vieira-Coelho, M. A., and Soares-da-Silva, P.
(1998) Uptake and intracellular fate of L-DOPA in a human intestinal
epithelial cell line: Caco-2, Am. J. Physiol., 275,
104-112.
15.Uchino, H., Kanai, Y., Kim, D. K., Wempe, M. F.,
Chairoungdua, A., Morimoto, E., Anders, M. W., and Endou, H. (2002)
Transport of amino acid-related compounds mediated by L-type amino acid
transporter 1 (LAT1): insights into the mechanisms of substrate
recognition, Mol. Pharmacol., 61, 729-737.
16.Khakimova, G. R., Kozina, E. A., Sapronova, A.
Ya., and Ugrumov, M. V. (2011) Dopamine release in the substantia nigra
and striatum at presymptomatic and early symptomatic stages in
parkinsonian mice, Neurochem. J., 5, 35-41.
17.Cartier, E. A., Parra, L. A., Baust, T. B.,
Quiroz, M., Salazar, G., Faundez, V., Egana, L., and Torres, G. E.
(2010) A biochemical and functional protein complex involving dopamine
synthesis and transport into synaptic vesicles, J. Biol. Chem.,
285, 1957-1966.
18.Okamura, H., Kitahama, K., Nagatsu, I., and
Geffard, M. (1988) Comparative topography of dopamine- and tyrosine
hydroxylase-immunoreactive neurons in the rat arcuate nucleus,
Neurosci. Lett., 95, 347-353.
19.Zoli, M., Agnati, L. F., Tinner, B., Steinbusch,
H. W. M., and Fuxe, K. (1993) Distribution of dopamine-immunoreactive
neurons and their relationships to transmitter and hypothalamic
hormone-immunoreactive neuronal systems in the rat mediobasal
hypothalamus. A morphometric and microdensitometric analysis, J.
Chem. Neuroanat., 6, 293-310.
20.Ershov, P. V., Ugrumov, M. V., Calas, A.,
Krieger, M., and Thibault, J. (2002) Differentiation of tyrosine
hydroxylase‐synthesizing and/or aromatic L‐amino acid
decarboxylase‐synthesizing neurons in the rat mediobasal
hypothalamus: quantitative double‐immunofluorescence study,
J. Comp. Neurol., 446, 114-122.
21.Meredith, G. E., Farrell, T., Kellaghan, P., Tan,
Y., Zahm, D. S., and Totterdell, S. (1999) Immunocytochemical
characterization of catecholaminergic neurons in the rat striatum
following dopamine‐depleting lesions, Eur. J. Neurosci.,
11, 3585-3596.
22.Lopez-Real, A., Rodriguez-Pallares, J., Guerra,
M. J., and Labandeira-Garcia, J. L. (2003) Localization and functional
significance of striatal neurons immunoreactive to aromatic L-amino
acid decarboxylase or tyrosine hydroxylase in rat Parkinsonian models,
Brain Res., 969, 135-146.
23.Betarbet, R., Turner, R., Chockkan, V., DeLong,
M. R., Allers, K. A., Walters, J., Levey, A. I., and Greenamyre, J. T.
(1997) Dopaminergic neurons intrinsic to the primate striatum, J.
Neurosci., 17, 6761-6768.
24.Palfi, S., Leventhal, L., Chu, Y., Ma, S. Y.,
Emborg, M., Bakay, R., Déglon, N., Hantraye, P., Aebischer, P.,
and Kordower, J. H. (2002) Lentivirally delivered glial cell
line-derived neurotrophic factor increases the number of striatal
dopaminergic neurons in primate models of nigrostriatal degeneration,
J. Neurosci., 22, 4942-4954.
25.Tande, D., Hoglinger, G., Debeir, T., Freundlieb,
N., Hirsch, E. C., and Francois, C. (2006) New striatal dopamine
neurons in MPTP-treated macaques result from a phenotypic shift and not
neurogenesis, Brain, 129, 1194-1200.