Omega-3 Polyunsaturated Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance Dexamethasone Sensitivity in Multiple Myeloma Cells by the p53/miR-34a/Bcl-2 Axis
Xianping Dai*, Mengshun Li, and Feng Geng
Binzhou Medical University, School of Pharmacy, Yantai, Shandong 264003, PR China; E-mail: rug104@163.com* To whom correspondence should be addressed.
Received March 30, 2017; Revision received April 20, 2017
Dexamethasone is widely used in multiple myeloma (MM) for its cytotoxic effects on lymphoid cells. However, many MM patients are resistant to dexamethasone, although some can benefit from dexamethasone treatment. In this study, we noted that ω-3 polyunsaturated fatty acids (PUFAs) enhanced the dexamethasone sensitivity of MM cells by inducing cell apoptosis. q-PCR analysis revealed that miR-34a could be significantly induced by PUFAs in U266 and primary MM cells. Transfection with miR-34a antagonist or miR-34a agomir could restore or suppress the dexamethasone sensitivity in U266 cells. Both luciferase reporter assay and Western blot showed that Bcl-2 is the direct target of miR-34a in MM cells. In addition, we observed that PUFAs induced p53 protein expression in MM cells under dexamethasone administration. Furthermore, suppressing p53 by its inhibitor, Pifithrin-α, regulated the miR-34a expression and modulated the sensitivity to dexamethasone in U266 cells. In summary, these results suggest that PUFAs enhance dexamethasone sensitivity to MM cells through the p53/miR-34a axis with a likely contribution of Bcl-2 suppression.
KEY WORDS: polyunsaturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, dexamethasone, apoptosis, miR-34a, p53, Bcl-2DOI: 10.1134/S0006297917070082
Abbreviations: Dex, dexamethasone; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MM, multiple myeloma; MTT (test), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; PFTa, Pifithrin-α; PI, propidium iodide; PUFAs, ω-3 polyunsaturated fatty acids; UTR, untranslated region.
Multiple myeloma (MM) is a hematological neoplasm characterized by
reasonless proliferation of malignant plasma cells and commonly
preceded by premalignant monoclonal gammopathy of undetermined
significance [1]. Treatment of MM has dramatically
improved since the introduction of novel therapies including proteasome
inhibitors, immunomodulatory drugs, and conventional chemical drugs.
However, MM remains an incurable disease because of tumor relapse. Drug
resistance plays a key role in MM relapse. The challenge for MM
treatment is not only the introduction of new drugs, but also
overcoming drug resistance.
Glucocorticoids including dexamethasone (Dex) are widely used in combination treatment with other drugs such as bortezomib and lenalidomide in MM patients [2]. Dex is efficacious in the treatment of hematological malignancies including MM by regulating the expression of genes involved in cell cycle and programmed cell death progression [3-5]. Unfortunately, numerous MM patients undergoing Dex treatment are or become insensitive to induction of cancer cell death [6]. Therefore, clarifying the mechanism of MM cell resistant to Dex is imperative.
The ω-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to enhance chemotherapeutic drug efficacy by inducing cancer cell apoptosis [7-12]. However, it is still unknown whether PUFAs contribute to Dex-sensitivity recovery in MM patients.
In this study, we observed that cotreatment with EPA/DHA and Dex remarkably reduced the growth of U266 cells, Dex-resistant MM cells, and at the same time induced the apoptosis of these cells. Furthermore, the expression of p53 and miR-34a were significantly increased, while the expression of Bcl-2 was reduced by combination treatment. We concluded that EPA and DHA enhance Dex sensitivity of MM cells through the p53/miR-34a/Bcl-2 axis.
MATERIALS AND METHODS
Cell culture. Human multiple myeloma cells U266 were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The cells were cultured in RPMI-1640 medium (HyClone, USA) with 10% fetal bovine serum (FBS; HyClone) at 37°C in a humidified atmosphere containing 5% CO2.
MTT assay. U266 cells and primary MM cells from two multiple myeloma patients, which were resistant to Dex, were seeded in 96-well plates (4·103 cells per well) at 37°C in an incubator containing 5% CO2. The cells were treated with 1 µM Dex, 50 µM EPA or DHA, and different doses of EPA/DHA (10, 30, or 50 µM) plus 1 µM Dex or Dex plus p53 inhibitor PFTa (Pifithrin α; SelleckChem, USA). Cell viability was tested using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma, USA) assay at 0, 12, 24, 36, and 48 h after treatment. Briefly, the cells were incubated with MTT at a final concentration of 0.5 mg/ml for 4 h. The supernatant was discarded, and the precipitated formazan was dissolved in dimethyl sulfoxide. Absorbance was measured at 570 nm with a microplate reader (Molecular Devices, USA). Further, we also added antagomir-34a, a miR-34a inhibitor (Suzhou GenePharma Co., Ltd., China), to EPA/DHA plus Dex groups to determine the functional role of miR-34a. Cell viability was tested after incubation for 24 h.
Flow cytometry. U266 cells and primary MM cells treated for 24 h with different drugs as described above were subjected to flow cytometry analysis using an Annexin V Apoptosis Detection Kit (Becton Dickinson, USA). EPA- or DHA-treated groups were considered as control. Cells were stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) for 25 min, and then they were analyzed using BD CantoII flow cytometer (BD BioSciences, USA). FACS data were analyzed using FlowJo software (Tree Star, Inc., USA).
Western blot. U266 cell lysates were prepared with RIPA lysis buffer (Beyotime, China) containing protease inhibitor cocktail (Roche, Switzerland). Protein samples were loaded for SDS-PAGE and transferred to a nitrocellulose membrane. After blockage with 5% fat-free milk, the membrane was probed with primary anti-caspase 3 (dilution 1 : 1000; Santa Cruz Biotechnology, USA), anti-Bcl-2 (dilution 1 : 2000; Santa Cruz Biotechnology), anti-p53 (dilution 1 : 2000; Santa Cruz Biotechnology), and anti-β-actin antibody (dilution 1 : 5000; Abcam, Great Britain). After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1 : 2000; Santa Cruz Biotechnology) for 1 h. The signal was visualized using the ECL detection system (Thermo Fisher, USA) and quantified by densitometry using Quantity One software (Bio-Rad, USA).
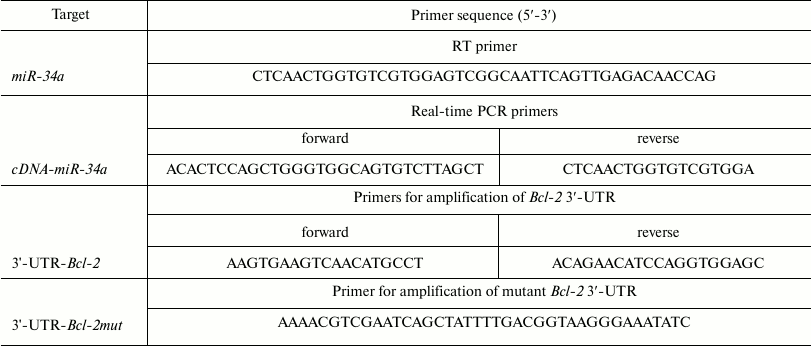
Quantitative real-time PCR. Total RNAs were extracted from U266 cells using RNAiso Plus reagent (TAKARA Biotechnology Co., Ltd., China). To detect miR-34a expression, total RNAs were reversed using MMLV reverse transcriptase with miR-34a-specific RT primer (see table). The resulting cDNA was then used as a template to perform real-time PCR using a Roche real-time PCR kit with specific PCR primers, forward and reverse (see the table). The transcripts were quantified by real-time PCR and normalized to the amount of GAPDH mRNA expression.
Luciferase reporter assay. The 3′-UTR of Bcl-2 (pMiR-Bcl2-WT) or mutant Bcl-2 (pMiR-Bcl2-mutant) was cloned and inserted downstream from the luciferase reporter gene. The sequences of primers (forward and reverse) for amplifying Bcl-2 3′-UTR and of primer for mutant Bcl-2 3′-UTR are given in the table. HEK293T cells were cotransfected with pMiR-Bcl2-WT or pMiR-Bcl2-mutant and miR-34a mimics or negative control (mimics) using Lipofectamine 2000. A Renilla luciferase-expressing plasmid pRL-TK (Promega, USA) used as control was also cotransfected. Cells were harvested and luciferase activity was determined using the Dual Luciferase Reporter Assay Kit (Promega) 24 h after transfection. The results are expressed as relative luciferase activity (firefly luciferase/Renilla luciferase).
Statistical analysis. Statistical analysis was performed for all experiments using Student’s t-test. All data are presented as mean ± SEM. Significant difference from control was taken at p < 0.05. All tests were done using Prism 6 software (GraphPad Prism 6).
Primers used in this study
RESULTS
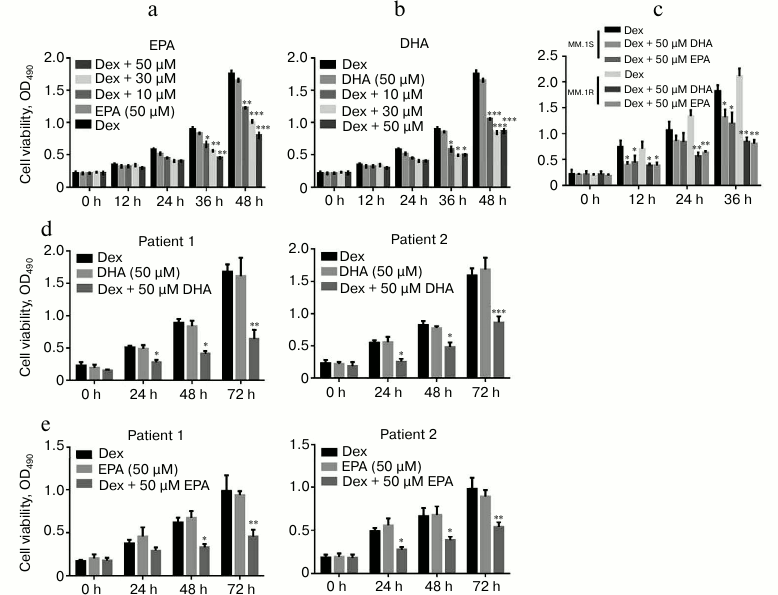
PFUAs enhanced Dex sensitivity in MM cells. Although many MM patients benefit from treatment with Dex, some patients are resistant to Dex. It has been reported that multiple myeloma cell line U266 is resistant to Dex [13]. To determine whether PUFAs contribute to improving Dex sensitivity in multiple myeloma, U266 cells were treated with EPA/DHA plus Dex. The cell growth was examined at 0, 12, 24, 36, and 48 h after incubation with EPA/DHA plus Dex by MTT assay. The results showed that EPA/DHA significantly enhanced the Dex sensitivity of MM cells in a time-dependent manner (Fig. 1, a and b). Additionally, with increased dose of EPA/DHA, the growth of U266 cells was suppressed. To confirm these results, we also used Dex-sensitive cells (MM.1S) and resistant cells (MM.1R) cells to perform these experiments. As expected, PUFAs slightly enhanced the Dex sensitivity in MM.1S cells but significantly elevated Dex sensitivity in MM.1R cells (Fig. 1c). Furthermore, two primary MM tumor cell cultures were also treated with EPA/DHA plus Dex, and cell growth was evaluated by MTT assays. As expected, PUFAs also enhanced the Dex sensitivity in primary MM cells (Fig. 1, d and e). Taken together, from these data we concluded that PUFAs improved the sensitivity to Dex in MM cells in time- and dose-dependent manner.
Fig. 1. EPA and DHA improved Dex sensitivity of MM cells. a, b) MTT assays were performed with U266 cells by treatment with different doses of EPA/DHA (10, 30, and 50 µM) and 1 µM Dex (50 µM EPA/DHA or Dex only were considered as controls). c) MTT assays were performed with MM.1S and MM.1R cells by treatment with 1 µM Dex and 50 µM EPA/DHA or 1 µM Dex. d, e) MTT assays were performed with two primary MM cell cultures after co-treatment with 50 µM EPA/DHA and 1 µM Dex. Data are shown as mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001.
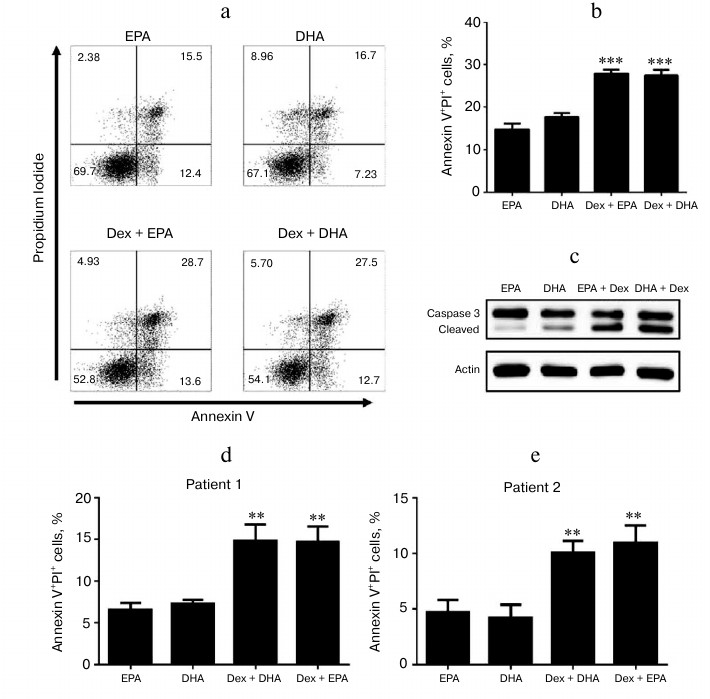
PUFAs induced MM cell apoptosis thus enhancing Dex sensitivity. It is widely accepted that PUFAs can induce cancer cell apoptosis. To determine how PUFAs enhanced the sensitivity to Dex, we next examined the apoptosis of MM cells after EPA/DHA plus Dex treatment. Flow cytometry analysis indicated that the combination EPA/DHA with Dex induced more apoptotic MM cells (Fig. 2, a and b). To further confirm these results, Western blot was performed for determination of the protein expression level of caspase-3 (Fig. 2c). Consistently, cleaved caspase-3 was significantly induced by PUFAs combined with Dex in U266 cells. In addition, two primary MM cell cultures were also subjected to flow cytometry analysis after incubation with PUFA plus Dex. As shown in Fig. 2 (d and e), PUFAs enhanced the apoptosis of the MM cells after Dex treatment. These observations imply that EPA/DHA enhanced the Dex sensitivity of MM cells by inducing cell apoptosis.
Fig. 2. EPA and DHA enhanced Dex sensitivity by inducing U266 cell apoptosis. a) Cell apoptosis was detected by flow cytometry in U266 cells treated with 50 µM EPA/DHA alone or 50 µM EPA/DHA plus 1 µM Dex for 24 h. The frequency of Annexin V+ PI+ U266 cells (a, b) and primary MM cells from two myeloma patients (d, e) was analyzed. c) The protein expression level of caspase-3 was determined by Western blot in U266 cells treated with 50 µM EPA/DHA alone or 50 µM EPA/DHA plus 1 µM Dex for 24 h. Data are presented as mean ± SEM; ** p < 0.01; *** p < 0.001.
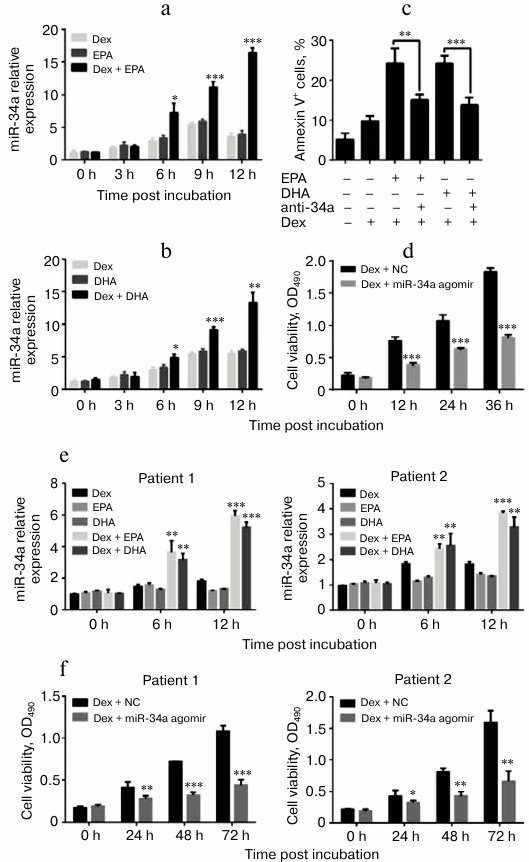
PUFAs induced expression of miR-34a, a tumor suppressor, thus enhancing Dex sensitivity in MM cells. MiR-34a has been widely reported as a tumor suppressor targeting CD44, Sirt1, or Bcl-2. To investigate whether the improvement of Dex sensitivity in MM cells was associated to miR-34a, quantitative PCR was performed for detecting the genomic expression level of miR-34a in MM cells treated with EPA/DHA alone or with added Dex. The results indicated that the co-administration of EPA/DHA with Dex significantly induced the expression of miR-34a (Fig. 3, a and b). Two primary MM cell cultures were also subject to q-PCR analysis for testing miR-34a expression after treatment with PUFA and Dex (Fig. 3e). To further investigate whether miR-34a played a functional role here, miR-34a was specifically suppressed by an inhibitor, miR-34a antagonist. As expected, the apoptosis of U266 cells induced by DHA/EPA plus Dex was dramatically recovered by miR-34a suppression (Fig. 3c). To confirm this conclusion, the level of miR-34a was upregulated by transfecting U266 cells with miR-34a agomir, and then the cell growth was detected. The MTT results indicated that miR-34a overexpression enhanced Dex sensitivity in U266 cells (Fig. 3d). These observations were also confirmed in two primary MM cell cultures (Fig. 3f). These results showed that EPA/DHA induced miR-34a (a tumor suppressor) expression thus enhancing Dex sensitivity in MM cells.
Fig. 3. EPA and DHA induced miR-34a expression in U266 cells. a, b) Expression of miR-34a detected in U266 cells by qRT-PCR at 0, 3, 9, and 12 h after treatment with 50 µM EPA/DHA, 1 µM Dex, or 50 µM EPA/DHA plus 1 µM Dex. c) Cell apoptosis was detected by flow cytometry of U266 cells treated as follows: medium, Dex, EPA/DHA plus Dex with/without antagonist-34a (anti-34a). d) MTT assays were performed with U266 cells treated with Dex/mimics (Dex + NC) or Dex + miR-34a agomir at 0, 12, 24, and 36 h after incubation. e) Expression of miR-34a in primary MM cells was analyzed after co-administration with PUFAs and Dex. f) MTT assays were performed in two primary MM cell cultures after co-administration with PUFAs and Dex. qRT-PCR results were normalized by β-actin. Data are presented as mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001.
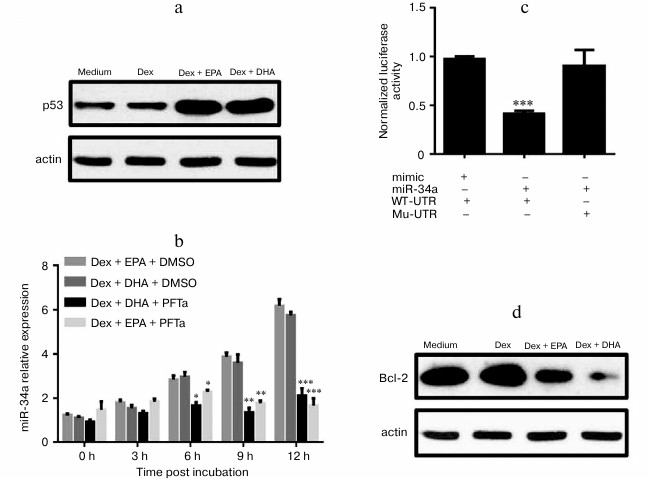
PUFAs increased Dex sensitivity in U266 cells via p53/miR-34a/Bcl-2 axis. It is well known that the expression of miR-34a is regulated by p53. As expected, p53 protein level was significantly increased in DHA/EPA plus Dex groups (Fig. 4a). These data indicated that DHA/EPA induced the expression of miR-34a to promote MM cell apoptosis. To confirm these observations, we also specifically suppressed p53 activity by incubating U266 cells with p53 inhibitor (Pifithrin-α, PFTa). The expression of miR-34a in U266 cells, which were co-administrated with PFTa and Dex/PUFAs, was determined. The q-PCR results indicated that p53 inhibition significantly suppressed the expression of miR-34a (Fig. 4b). To further explore the functional target of miR-34a here, luciferase reporter assay was performed (Fig. 4c). Bcl-2 is a target of miR-34a and a suppressor of cell apoptosis as well. The expression of Bcl-2 was examined by Western blot. Consistently, the level of Bcl-2 was reduced in DHA/EPA with Dex groups (Fig. 4d). All these observations led us to conclusion that PUFAs enhance Dex sensitivity in MM cells via the p53/miR-34a/Bcl-2 axis.
Fig. 4. DHA and EPA enhanced Dex sensitivity in U266 cells via the p53/miR-34a/Bcl-2 axis. Protein level of p53 (a) and Bcl-2 (d) was determined by Western blot in U266 cells treated with the medium, 50 µM EPA or DHA, 50 µM EPA/DHA, plus 1 µM Dex. b) Expression of miR-34a was determined at 0, 3, 9, and 12 h after incubation of U266 cells, which were treated by 50 µM DHA/EPA and 1 µM Dex with/without 1 µM p53 inhibitor (PFTa). c) Luciferase reporter assay was performed to determine whether Bcl-2 was the direct target of miR-34a in U266 cells. Data are shown as mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001.
DISCUSSION
In this study, we found that PUFAs increased myeloma cell sensitivity to Dex by prompting cell apoptosis. The MM cell line U266 has been reported to have the capacity for Dex insensitivity [13]. In vitro, cell proliferation assay showed that EPA or DHA displayed a comparable inhibitory role with Dex in U266 cells. These data indicated PUFAs alone were insufficient to suppress U266 cell growth in vitro. The mechanism of cancer cell suppression by PUFAs includes induction of apoptosis [14, 15], lipid peroxidation causing irreversible cell damage [16-18], and modulation of gene expression including that of transcription factors [19-22]. According to the results of MTT assays, EPA or DHA alone induced moderate U266 cell apoptosis but was insufficient to suppress cell growth without Dex treatment. These data demonstrated that PUFAs not only induced cell apoptosis but also enhanced Dex sensitivity of U266 cells. Other investigators have also reported that PUFAs enhanced Dex sensitivity to suppress multiple myeloma cell growth [23]. It is unclear if combination treatment with PUFAs and Dex induces other processes besides inducing cell apoptosis to suppress U266 cell growth in vitro.
A microRNA is a small noncoding RNA binding to the 3′UTR of mRNAs to repress the protein expression [24]. Recently, microRNAs were reported to be mediators in biological processes including tumorigenesis [25]. It has been reported that several microRNAs were differently expressed in MM patients, such as miR-221/222, -181a, -181b, -32, -17-92, etc. [26]. We found that treatment with EPA/DHA and Dex reinforced gene expression level of miR-34a in U266 cell compared with EPA/DHA or Dex treatment alone. It is well known that miR-34a acts as a tumor suppressor by targeting Bcl-2, Sirt1, and CDK6 [27]. In this study, the expression of mi-R-34a was significantly increased in the EPA or DHA with Dex group. Further, cell viability was restored by miR-34a inhibition. We concluded a combination treatment with EPA/DHA and Dex regulated miR-34a expression, although the detailed mechanisms are unknown.
We know miR-34a is a p53 direct transcription target gene [27] and acts as a tumor suppressor [28], though it is dispensable for p53 tumor suppression [29]. Our study indicated that p53 was induced by EPA or DHA with Dex treatment in U266 cells. This result suggested that EPA/DHA enhanced Dex sensitivity to U266 cells by p53 expression stimulation and extensively increased miR-34a expression level. Bcl-2, a direct target of miR-34a, was reported to suppress cell apoptosis [30, 31]. Interestingly, the expression of Bcl-2 was significantly reduced in EPA/DHA with the Dex-treated groups. We conclude here that PUFAs improve Dex sensitivity of U266 cells via p53/miR-34a/Bcl-2 axis. Whether EPA and DHA have an effect on other molecules associated to miR-34a expression is not clear, and this will be investigated in our next research programs. Moreover, whether there is any other target for p53-induced miR-34a in MM cells will be studied in our future investigations. Furthermore, the p53/miR-34a/Bcl-2 axis is still an alternative elucidation for the enhancement of Dex sensitivity to U266 cells treated with EPA or DHA.
REFERENCES
1.Kyle, R. A., Therneau, T. M., Rajkumar, S. V.,
Offord, J. R., Larson, D. R., Plevak, M. F., and Melton, L. J., 3rd.
(2002) A long-term study of prognosis in monoclonal gammopathy of
undetermined significance, N. Engl. J. Med., 346,
564-569.
2.Chesi, M., and Bergsagel, P. L. (2013) Molecular
pathogenesis of multiple myeloma: basic and clinical updates, Int.
J. Hematol., 97, 313-323.
3.Frankfurt, O., and Rosen, S. T. (2004) Mechanisms
of glucocorticoid-induced apoptosis in hematologic malignancies:
updates, Curr. Opin. Oncol., 16, 553-563.
4.Renner, K., Ausserlechner, M. J., and Kofler, R.
(2003) A conceptual view on glucocorticoid-lnduced apoptosis, cell
cycle arrest and glucocorticoid resistance in lymphoblastic leukemia,
Curr. Mol. Med., 3, 707-717.
5.Schmidt, S., Rainer, J., Ploner, C., Presul, E.,
Riml, S., and Kofler, R. (2004) Glucocorticoid-induced apoptosis and
glucocorticoid resistance: molecular mechanisms and clinical relevance,
Cell Death Differ., 11, Suppl. 1, S45-55.
6.Gross, K. L., Lu, N. Z., and Cidlowski, J. A.
(2009) Molecular mechanisms regulating glucocorticoid sensitivity and
resistance, Mol. Cell. Endocrinol., 300, 7-16.
7.Biondo, P. D., Brindley, D. N., Sawyer, M. B., and
Field, C. J. (2008) The potential for treatment with dietary long-chain
polyunsaturated n-3 fatty acids during chemotherapy, J. Nutr.
Biochem., 19, 787-796.
8.De Aguiar Pastore Silva, J., Emilia de Souza Fabre,
M., and Waitzberg, D. L. (2015) Omega-3 supplements for patients in
chemotherapy and/or radiotherapy: a systematic review, Clin.
Nutr., 34, 359-366.
9.Hajjaji, N., and Bougnoux, P. (2013) Selective
sensitization of tumors to chemotherapy by marine-derived lipids: a
review, Cancer Treat. Rev., 39, 473-488.
10.Merendino, N., Costantini, L., Manzi, L.,
Molinari, R., D’Eliseo, D., and Velotti, F. (2013) Dietary
omega-3 polyunsaturated fatty acid DHA: a potential adjuvant in the
treatment of cancer, Biomed Res. Int., 310186.
11.Siddiqui, R. A., Harvey, K. A., Xu, Z.,
Bammerlin, E. M., Walker, C., and Altenburg, J. D. (2011)
Docosahexaenoic acid: a natural powerful adjuvant that improves
efficacy for anticancer treatment with no adverse effects,
Biofactors, 37, 399-412.
12.Wang, J., Luo, T., Li, S., and Zhao, J. (2012)
The powerful applications of polyunsaturated fatty acids in improving
the therapeutic efficacy of anticancer drugs, Expert Opin. Drug
Deliv., 9, 1-7.
13.Kervoelen, C., Menoret, E., Gomez-Bougie, P.,
Bataille, R., Godon, C., Marionneau-Lambot, S., Moreau, P.,
Pellat-Deceunynck, C., and Amiot, M. (2015) Dexamethasone-induced cell
death is restricted to specific molecular subgroups of multiple
myeloma, Oncotarget, 6, 26922-26934.
14.Chiu, L. C., Wong, E. Y., and Ooi, V. E. (2004)
Docosahexaenoic acid modulates different genes in cell cycle and
apoptosis to control growth of human leukemia HL-60 cells, Int. J.
Oncol., 25, 737-744.
15.Shirota, T., Haji, S., Yamasaki, M., Iwasaki, T.,
Hidaka, T., Takeyama, Y., Shiozaki, H., and Ohyanagi, H. (2005)
Apoptosis in human pancreatic cancer cells induced by eicosapentaenoic
acid, Nutrition, 21, 1010-1017.
16.Colas, S., Maheo, K., Denis, F., Goupille, C.,
Hoinard, C., Champeroux, P., Tranquart, F., and Bougnoux, P. (2006)
Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma
to anthracycline: a role for tumor vascularization, Clin. Cancer
Res., 12, 5879-5886.
17.Sturlan, S., Baumgartner, M., Roth, E., and
Bachleitner-Hofmann, T. (2003) Docosahexaenoic acid enhances arsenic
trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells,
Blood, 101, 4990-4997.
18.Vibet, S., Maheo, K., Gore, J., Dubois, P.,
Bougnoux, P., and Chourpa, I. (2007) Differential subcellular
distribution of mitoxantrone in relation to chemosensitization in two
human breast cancer cell lines, Drug Metab. Dispos., 35,
822-828.
19.Daak, A. A., Elderdery, A. Y., Elbashir, L. M.,
Mariniello, K., Mills, J., Scarlett, G., Elbashir, M. I., and
Ghebremeskel, K. (2015) Omega-3 (n-3) fatty acids down-regulate nuclear
factor-kappa B (NF-kappaB) gene and blood cell adhesion molecule
expression in patients with homozygous sickle cell disease, Blood
Cells Mol. Dis., 55, 48-55.
20.Lee, J. Y., Zhao, L., Youn, H. S., Weatherill, A.
R., Tapping, R., Feng, L., Lee, W. H., Fitzgerald, K. A., and Hwang, D.
H. (2004) Saturated fatty acid activates but polyunsaturated fatty acid
inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1,
J. Biol. Chem., 279, 16971-16979.
21.Schley, P. D., Jijon, H. B., Robinson, L. E., and
Field, C. J. (2005) Mechanisms of omega-3 fatty acid-induced growth
inhibition in MDA-MB-231 human breast cancer cells, Breast Cancer
Res. Treat., 92, 187-195.
22.Wang, Y., Lin, Q. W., Zheng, P. P., Zhang, J. S.,
and Huang, F. R. (2013) DHA inhibits protein degradation more
efficiently than EPA by regulating the PPARgamma/NFkappaB pathway in
C2C12 myotubes, Biomed. Res. Int., 2013, 318981.
23.Wu, S. H., Zheng, C. P., Chen, S. Y., Liu, Z.,
Lin, B. J., Fan, Y. F., and Weng, S. S. (2016) Effects of omega-3
polyunsaturated fatty acids on multiple myeloma growth inhibition and
enhanced sensitivity of dexamethasone, Zhonghua Xue Ye Xue Za
Zhi, 37, 1085-1088.
24.Bartel, D. P. (2004) MicroRNAs: genomics,
biogenesis, mechanism, and function, Cell, 116,
281-297.
25.Cheng, C. J., Bahal, R., Babar, I. A., Pincus,
Z., Barrera, F., Liu, C., Svoronos, A., Braddock, D. T., Glazer, P. M.,
Engelman, D. M., Saltzman, W. M., and Slack, F. J. (2015) MicroRNA
silencing for cancer therapy targeted to the tumour microenvironment,
Nature, 518, 107-110.
26.Rossi, M., Tagliaferri, P., and Tassone, P.
(2015) MicroRNAs in multiple myeloma and related bone disease, Ann.
Transl. Med., 3, 334.
27.Misso, G., Di Martino, M. T., De Rosa, G.,
Farooqi, A. A., Lombardi, A., Campani, V., Zarone, M. R., Gulla, A.,
Tagliaferri, P., Tassone, P., and Caraglia, M. (2014) Mir-34: a new
weapon against cancer? Mol. Ther. Nucleic Acids, 3,
e194.
28.Hermeking, H. (2010) The miR-34 family in cancer
and apoptosis, Cell Death Differ., 17, 193-199.
29.Concepcion, C. P., Han, Y. C., Mu, P., Bonetti,
C., Yao, E., D’Andrea, A., Vidigal, J. A., Maughan, W. P.,
Ogrodowski, P., and Ventura, A. (2012) Intact p53-dependent responses
in miR-34-deficient mice, PLoS Genet., 8, e1002797.
30.Hui, K., Yang, Y., Shi, K., Luo, H., Duan, J.,
An, J., Wu, P., Ci, Y., Shi, L., and Xu, C. (2014) The p38
MAPK-regulated PKD1/CREB/Bcl-2 pathway contributes to selenite-induced
colorectal cancer cell apoptosis in vitro and in vivo,
Cancer Lett., 354, 189-199.
31.Song, H. Y., Deng, X. H., Yuan, G. Y., Hou, X.
F., Zhu, Z. D., Zhou, L., and Ren, M. X. (2014) Expression of bcl-2 and
p53 in induction of esophageal cancer cell apoptosis by ECRG2 in
combination with cisplatin, As. Pac. J. Cancer Prev., 15,
1397-1401.