Expression of Hormonal Carcinogenesis Genes and Related Regulatory microRNAs in Uterus and Ovaries of DDT-Treated Female Rats
T. S. Kalinina1,2*, V. V. Kononchuk1, and L. F. Gulyaeva1,2
1Institute of Molecular Biology and Biophysics, 630117 Novosibirsk, Russia; E-mail: tatyanasergeevna12@mail.ru2Novosibirsk State University, 630090 Novosibirsk, Russia
* To whom correspondence should be addressed.
Received June 21, 2017; Revision received July 10, 2017
The insecticide dichlorodiphenyltrichloroethane (DDT) is a nonmutagenic xenobiotic compound able to exert estrogen-like effects resulting in activation of estrogen receptor-α (ERα) followed by changed expression of its downstream target genes. In addition, studies performed over recent years suggest that DDT may also influence expression of microRNAs. However, an impact of DDT on expression of ER, microRNAs, and related target genes has not been fully elucidated. Here, using real-time PCR, we assessed changes in expression of key genes involved in hormonal carcinogenesis as well as potentially related regulatory oncogenic/tumor suppressor microRNAs and their target genes in the uterus and ovaries of female Wistar rats during single and chronic multiple-dose DDT exposure. We found that applying DDT results in altered expression of microRNAs-221, -222, -205, -126a, and -429, their target genes (Pten, Dicer1), as well as genes involved in hormonal carcinogenesis (Esr1, Pgr, Ccnd1, Cyp19a1). Notably, Cyp19a1 expression seems to be also regulated by microRNAs-221, -222, and -205. The data suggest that epigenetic effects induced by DDT as a potential carcinogen may be based on at least two mechanisms: (i) activation of ERα followed by altered expression of the target genes encoding receptor Pgr and Ccnd1 as well as impaired expression of Cyp19a1, affecting, thereby, cell hormone balance; and (ii) changed expression of microRNAs resulting in impaired expression of related target genes including reduced level of Cyp19a1 mRNA.
KEY WORDS: DDT, ERα, hormonal carcinogenesis, microRNAsDOI: 10.1134/S0006297917100042
Abbreviations: DDT, dichlorodiphenyltrichloroethane; ERα, estrogen receptor-α; miR, microRNA; PGR, progesterone receptor.
In many cell types, estrogen receptors (ER) and progesterone receptors
(PGR) mediate responses to steroid hormones. High ER level is found in
the uterus, mammary gland, Fallopian tubes, and, to a lesser extent, in
prostate, ovaries, hypophysis, and liver [1]. For
PGR, like ER, it is known that it has a similar pattern of expression,
most abundantly being found in the uterus, ovaries, Fallopian tubes,
and mammary gland (The Human Protein Atlas, http://www.proteinatlas.org).
Such receptors mediate hormone-induced production of mRNAs encoding
proteins, many of which regulate cell proliferation and differentiation
in the target organs. Altering expression or activity of ER and
PGR genes may result in tumor transformation, mainly in organs
where they are expressed at high level [2-4]. Usually, such alterations are related either to
changes in hormone concentration or effects induced by xenobiotics. The
latter may exhibit estrogen-like effects via various mechanisms: (i) by
directly activating ER after binding or indirectly acting via other
transcription factors; and (ii) resulting in changed level of activated
ER inside the cells, by affecting its expression or enzymes involved in
estrogen production [1, 5].
Many chemical compounds such as phytoestrogens and bisphenol A used in
production of certain plastics can activate ER [6].
In addition, ER activity may be changed due to organochlorine
pesticides, metabolites of polychlorinated biphenyls generally
characterized by high stability and accumulation in food chains [1, 7]. Currently, production of
organochlorine pesticides is significantly limited, but due to their
high stability and wide use in the past in agriculture and in combating
diseases such as malaria and pediculosis, people are still exposed to
their activity [8, 9]. Among
these pesticides, dichlorodiphenyltrichloroethane (DDT) holds a lead
position in terms of its volume of output and scale of application [10]. DDT has been proved a carcinogen in rodents [11], and in primates its long-term administration
also resulted in structural and functional changes in the liver [9]. Data regarding carcinogenicity of DDT in humans
require further confirmation [10]. The effects
triggered by DDT are due to activated nuclear receptors CAR, PXR, and
ERα [1, 12, 13] with subsequently altered expression of related
target genes regulating cell cycle, inducing cell proliferation and
thus resulting in elevated risk of developing tumors [14]. For instance, the Ccnd1 gene encoding an
important regulator of the G1/S-transition in cell cycle is a common
target for both ER and CAR. Upregulated expression of the Ccnd1
gene contributes to cancer development [15], and
by inducing its transcription by DDT via both receptors, it thus
becomes one of the major targets for this xenobiotic agent pointing at
carcinogenic properties of DDT both in liver and tissues that are
characterized by high ERα expression level. It is noteworthy
that, apart from directly acting on ERα, DDT metabolites were
found to exert activating effects on aromatase (CYP19A1) involved in
estrogen production, which might result in upregulated generation of
estradiol and, subsequently, uncontrolled ERα activation [16, 17], thus facilitating
hormonal carcinogenesis. Moreover, recent studies suggest that DDT may
affect the profile of microRNA (miR) expression [18, 19]. In turn, impaired
microRNA expression acts in parallel with development of many diseases
including malignant transformation, where microRNAs may regulate
translation of oncogenes or tumor growth suppressor genes [20, 21]. Importantly, by acting
via microRNA expression, DDT may also influence the proteins encoded by
target genes controlled by ER or PGR, because for a set of microRNAs an
inhibitory effect on expression of these receptors was observed. For
instance, miR-221 and -222, usually displaying oncogenic functions in
cancer [22, 23], target the
gene ESR1 encoding ERα [24], whereas
Pgr was shown to be targeted by miR-126a in mice [25]. However, an impact of DDT on expression of the
ER, microRNAs, and related target genes has not been fully elucidated.
Therefore, we investigated the impact of single and chronic
multiple-dose DDT exposure on expression of the key genes involved in
hormonal carcinogenesis, such as Esr1, Pgr,
Cyp19a1, and Ccnd1, as well as certain oncogenic/tumor
suppressor microRNAs that were predicted or experimentally shown to
target Esr1, Pgr, and Cyp19a1 in the uterus and
ovaries of female Wistar rats. To assess the role of microRNA affected
by DDT in disturbing expression of the predicted target hormonal
carcinogenesis genes in humans, we examined microRNAs whose expression
changes during malignant transformation occurring in organs with high
ER and PGR expression (mammary gland, uterus, ovaries) [26-28]. Thus, we evaluated the
effect of DDT on miR-21 level, whose targets were predicted to be human
Esr1 and Cyp19a1, miR-221, -222, and -126a as regulators
of ER and PGR expression as well as miR-205 and miR-429 predicted to
target Cyp19a1 and Pgr. In addition, the Cyp19a1
gene is a predicted target for miR-221 and -222. A role for such
microRNAs in carcinogenesis has been extensively examined, and their
expression was shown to be impaired in many tumor types [29-32]; therefore, investigating
the action of chemical compounds on expression of these microRNAs and
related target genes including those involved in hormonal
carcinogenesis is of importance for understanding mechanisms of
xenobiotic-associated carcinogenic activity. The level of mRNA for the
Cdkn1b, Pten, Itga6, Slc7a5, Dicer1,
and Bcl2 genes targeted by the examined microRNAs was
investigated to demonstrate DDT-elicited effects.
MATERIALS AND METHODS
Chemicals and animals. p,p′-DDT (2,2-dichlorodiphenyl-1,1,1-trichloroethane) was purchased from Sigma-Aldrich (USA). Analytical grade chemicals and solvents were obtained from commercial sources. Sexually mature female Wistar rats, 150-200 g, were obtained from the Federal Research Center Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences. The rats were housed under standard setting of humidity, temperature (22 ± 2°C), and light-dark cycle (12 h : 12 h), in specific pathogen-free conditions, with food and water ad libitum. All experimental procedures were approved by the Bioethics Committee of the Institute of Molecular Biology and Biophysics.
Experimental groups. The rats were divided into five groups with five female rats in each group: group 1) DDT-in-oil, administered once a week via intraperitoneal route for 12 weeks, 50 mg/kg; group 2) DDT-in-oil, administered once a week via intraperitoneal route for 12 weeks, 10 mg/kg; group 3) vehicle oil administered once a week via intraperitoneal route for 12 weeks (control); group 4) DDT-in-oil, administered via intraperitoneal route, single dose 75 mg/kg; group 5) vehicle oil administered via intraperitoneal route, single dose (control). The rats were sacrificed 72 h after the last injection.
RNA isolation and cDNA synthesis. Total RNA was isolated using the RNeasy® Lipid Tissue Mini Kit (Qiagen, USA) according to the manufacturer’s protocol. RNA integrity was checked by running agarose gel electrophoresis. RNA concentration and purity were assessed using an Agilent-8453 spectrophotometer (Agilent Technologies, USA) at wavelengths of 260 and 280 nm. Reverse transcription was performed by using the OT-M-MuLV-RH kit (BiolabMix, Russia) according to the manufacturer’s protocol. One microgram of RNA was used for RT-PCR reaction.
Real-time PCR. cDNA was used in real-time PCR to measure level of mRNA for the Esr1, Pgr, Ccnd1, Cyp19a1, Cdkn1b, Pten, Dicer1, Slc7a5, Itga6, and Bcl2 genes by adding a BioMaster HS-qPCR SYBR Blue(2×) (BiolabMix) reaction mix, followed by applying the CFX96™ Detection System (Bio-Rad Laboratories, USA). Gapdh, Polr2a, and 18S rRNA were used as reference genes. Specific primers used in the study are shown in Table 1.
Table 1. Primer sequence for mRNA real-time
RT-PCR
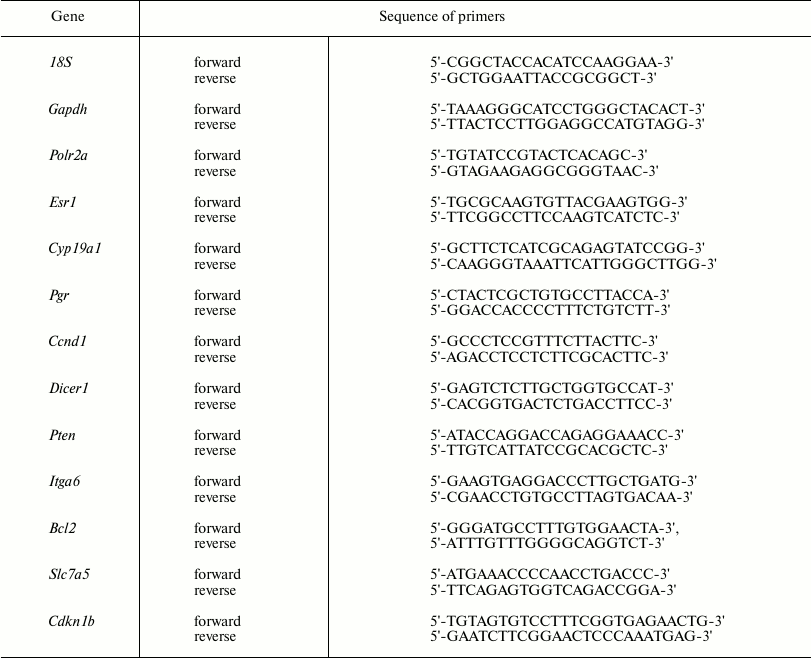
The optimal concentration for each primer was 300 nM.
Each PCR reaction was performed by using 0.3 µl cDNA, at final volume 25 µl, under the following conditions: initial denaturation for 5 min at 95°C, followed by 40 cycles: denaturation for 15 s at 95°C, annealing for 20 s at 60°C, elongation and fluorescence data processing for 30 s at 72°C. Melting profiles were used to assess PCR specificity. In each experiment, analyzed cDNA added with primers specific to the target and reference genes were placed in the same 96-well plate (in triplicate for each sample). Relative gene expression level was assessed based on threshold cycle (Ct) values considering PCR efficacy (E) for both the analyzed and reference genes.
RNA isolation for measuring microRNA expression. To isolate RNA, 50 mg tissue sample was combined with 500 µl of guanidine lysis buffer (4 M guanidine isothiocyanate, 25 mM sodium citrate, 0.3% sarcosyl, 0.1% 2-mercaptoethanol, 25 mM CH3COONa). Then, the solution was mixed and incubated for 10 min at 65°C. Samples were centrifuged for 2 min at 10,000g. Finally, supernatant was combined with an equal volume of isopropanol, mixed, incubated for 5 min at room temperature, and centrifuged for 10 min at 10,000g. After that, the supernatant was decanted, and the pellet was washed out with 500 µl of 70% EtOH and 300 µl of acetone, dried, and dissolved in 200 µl of mQ-H2O.
Measurement of microRNA expression level. Relative expression level for microRNAs-21, -221, -222, -429, -205, and -126a was measured using real-time reverse transcription-PCR (RT-PCR). A reverse transcription reaction was performed using stem-loop-primers and an OT-M-MuLV-RH kit (BiolabMix) according to the manufacturer’s protocol. Real-time PCR was performed using TaqMan-probes and PCR kits together with BioMaster UDG HS-qPCR (2×) fluorescence probes (BiolabMix) according to the manufacturer’s protocol. To detect PCR products, a CFX96™ Detection System (Bio-Rad Laboratories) was applied. Small nuclear RNA U6 and U48 were used to normalize the data.
Primers used for reverse transcription reaction for microRNAs are shown in Table 2.
Table 2. Primer sequence for microRNA
real-time RT-PCR
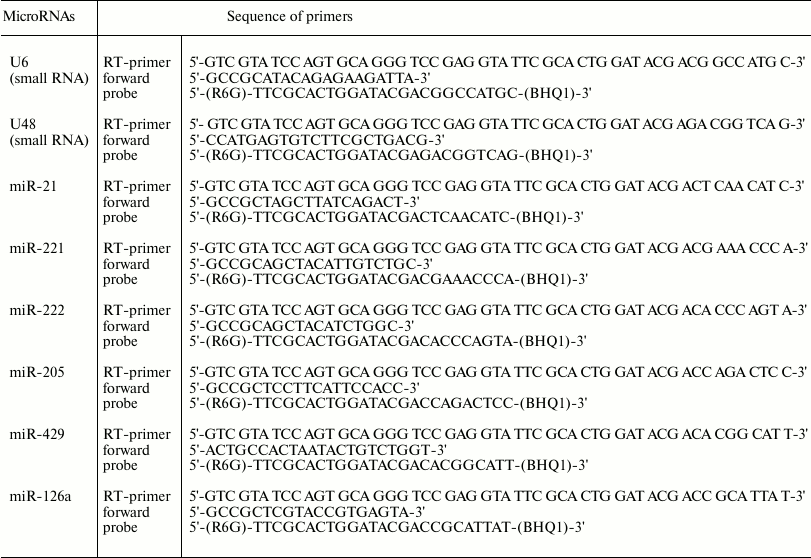
In each experiment, analyzed cDNA together with primers specific to the target and reference snRNA were placed in the same 96-well plate (in triplicate for each sample). Relative expression level was assessed based on threshold cycle (Ct) values considering PCR efficacy (E) for both the analyzed and reference genes. Specific primers for real-time PCR are shown in Table 2. Similar type of reverse primer targeting the stem-loop region in the synthesized cDNAs was as follows: 5′-AGT GCA GGG TCC GAG GTA-3′.
Bioinformatics analysis. Algorithms TargetScan, miRanda, miRDB, and microRNA Data Integration Portal (mirDIP) database were applied to predict microRNA target genes.
Statistical analysis. The data are displayed as mean and standard deviation (M ± SD). Two groups were compared using the unpaired two-tailed Student’s t-test. Significance level was set at p < 0.05.
RESULTS
Effect of DDT on expression of target genes related to hormonal carcinogenesis. To examine the mechanism of DDT action, the relative mRNA level for genes Esr1 and Cyp19a1 as well as ERα target genes Pgr and Ccnd1 playing an important role in regulating cell proliferation and differentiation was measured in uterus and ovaries isolated from female rats after applying a single dose DDT (75 mg/kg) or chronic 12-week treatment (at dose of 10 and 50 mg/kg).
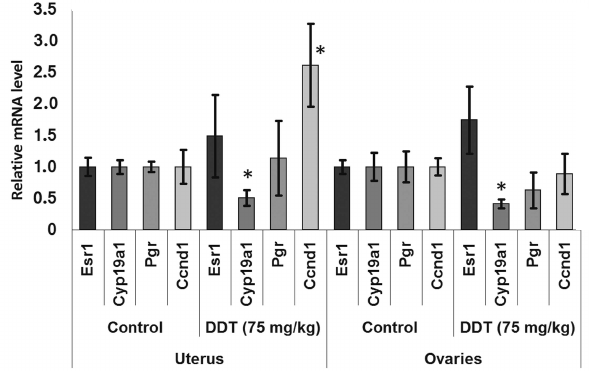
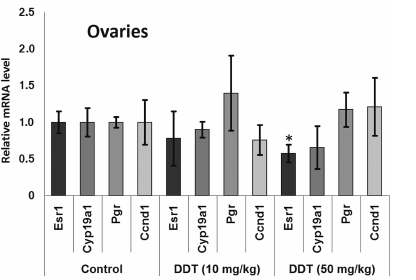
After a single DDT exposure, we found Cyp19a1 expression in the ovaries was decreased 2-fold (Fig. 1). When the rats were chronically treated with DDT, the level of Cyp19a1 mRNA in ovaries tended to decline, but no significant differences were documented. The relative level of Esr1 expression in the ovaries of the rats chronically treated with high-dose DDT was downregulated 2-fold (Fig. 2). Despite a reduced ERα expression, the level of Pgr and Ccnd1 mRNA did not change, perhaps due to a DDT-activating effect on this receptor.
Fig. 1. Relative mRNA level of genes involved in hormonal carcinogenesis in uterus and ovaries of single-dose-DDT-treated female rats. Y-axis: Treatment/Control mRNA ratio is depicted. The data are displayed as mean ± SD (n = 5) (shown for all figures). * Statistical significance in treatment vs. control group (p < 0.05) (shown for all figures).
Fig. 2. Relative mRNA level of genes involved in hormonal carcinogenesis in ovaries from chronically DDT-treated female rats. Y-axis: Treatment/Control mRNA ratio is depicted.
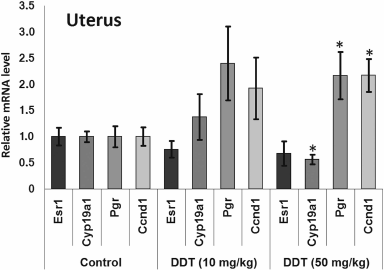
The level of Cyp19a1 expression in the uterus was decreased 2-fold both after a single dose and after high-dose chronic DDT exposure (Figs. 1 and 3). No changes for mRNA Esr1 level were detected in the uterus. Expression of the ERα target genes Pgr and Ccnd1 was upregulated 2-fold after chronic high-dose DDT treatment, suggesting that ERα was activated. After a single dose exposure to DDT, no change in mRNA Pgr expression was found in the uterus, but, in contrast, Ccnd1 expression was upregulated by 2.6-fold.
Fig. 3. Relative mRNA level of genes involved in hormonal carcinogenesis in the uterus from chronically DDT-treated female rats. Y-axis: Treatment/Control mRNA ratio is depicted.
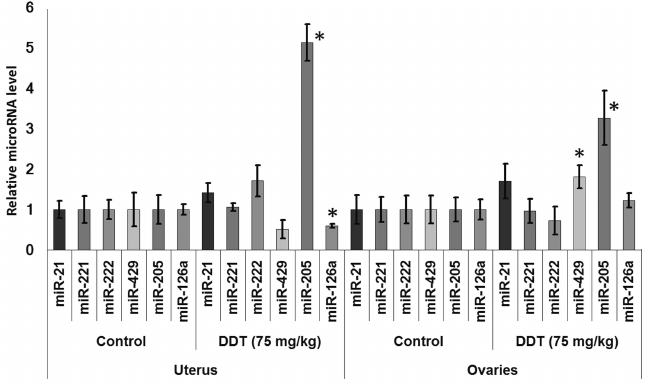
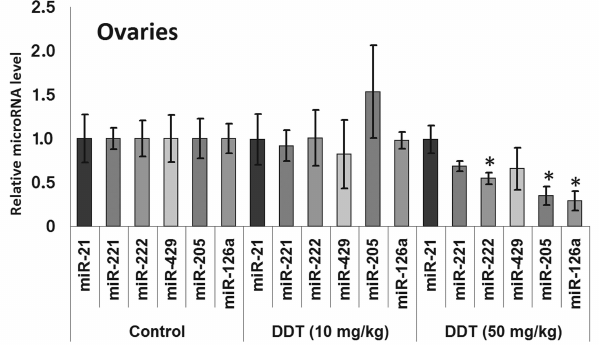
Changes in expression of oncogenic and tumor suppressor microRNAs after DDT exposure. To assess an effect of DDT on microRNA expression, real-time RT-PCR was conducted to measure relative expression of a set of oncogenic and tumor suppressor microRNAs. We found that a single-dose DDT exposure upregulated expression of miR-429 and miR-205 in ovaries by 2- and 3-fold, respectively (Fig. 4). However, no significant increase was detected in the level of these microRNAs after chronic DDT exposure vs. untreated rats, but after a chronic high-dose DDT treatment the level of ovarian miR-222 as well as miR-126a and miR-205 was downregulated by 2- and 3-fold, respectively (Fig. 5).
Fig. 4. Relative level of oncogenic/tumor suppressor microRNAs in the uterus and ovaries of single-dose-DDT-treated female rats. Y-axis: Treatment/Control microRNA ratio is depicted.
Fig. 5. Relative level of oncogenic/tumor suppressor microRNAs in the ovaries from chronically DDT-treated female rats. Y-axis: Treatment/Control microRNA ratio is depicted.
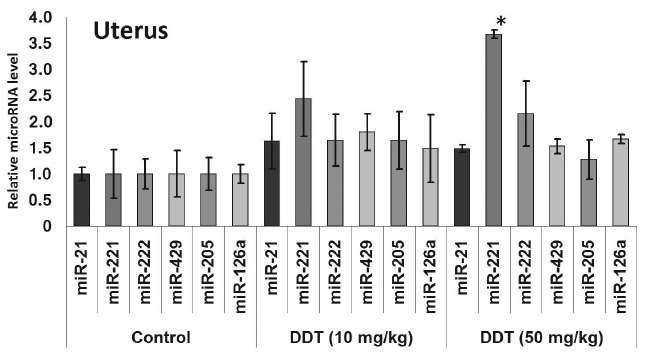
In the uterus, a single DDT exposure upregulated expression of miR-205 by 5-fold and reduced relative expression of miR-126a by 1.7-fold (Fig. 4). On the other hand, chronic exposure to a high-dose of DDT led to elevated uterine miR-221 expression by 3.5-fold (Fig. 6).
Fig. 6. Relative level of oncogenic/tumor suppressor microRNAs in the uterus from chronically DDT-treated female rats. Y-axis: Treatment/Control microRNA ratio is depicted.
No change in miR-21 expression was observed after single or chronic DDT exposure.
After performing bioinformatics analysis, it was demonstrated that in both rats and humans the examined microRNAs might target hormonal carcinogenesis genes affected after DDT exposure (Table 3).
Table 3. MicroRNAs and their potential
target genes involved in hormonal carcinogenesis (similar human and rat
microRNAs are highlighted in bold)
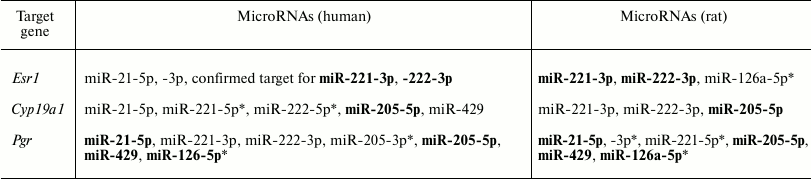
Thus, it seems that the downregulated expression of Cyp19a1 mRNA found both in the uterus and ovaries of DDT-treated female rats is related to upregulated expression of miR-221 and miR-205.
Effects of DDT on expression of microRNA target genes. Next, to confirm the effects of DDT on expression of the examined microRNAs, we checked expression of their relevant target genes. To do this, a set of genes with frequently altered expression in cancer cells was selected: 1) Cdkn1b, Dicer1 – potential target genes for miR-221 and -222; 2) Pten, Bcl2 – potential target genes for miR-205, -429, -221, and -222; 3) Itga6, Slc7a5 – potential target genes for miR-126a.
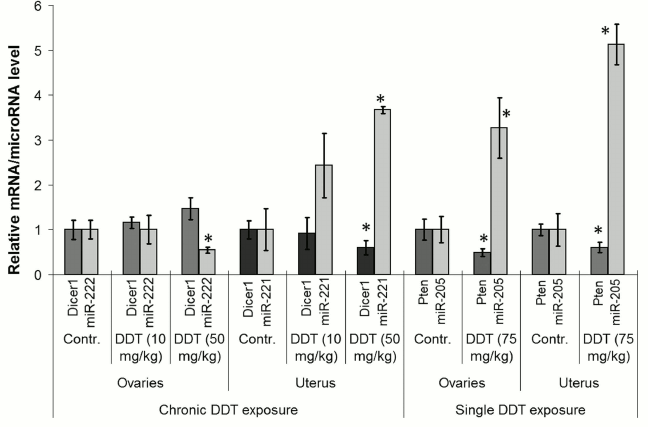
Among them, the level of Pten and Dicer1 mRNA expression was significantly changed. For instance, the level of ovarian and uterine Pten mRNA was found to be significantly downregulated by 2-fold after a single-dose DDT exposure, whereas for Dicer1 mRNA uterine expression was decreased by 2-fold after chronic exposure (Fig. 7). Such alterations in expression of the target genes were in inverse relationship with the level of microRNAs, where the former may be potentially targeted by the latter (miR-205 and miR-221, respectively).
Fig. 7. Comparison of microRNA level and related target genes in the uterus and ovaries of female DDT-treated rats. Y-axis: mRNA/microRNA ratio in Treatment vs. Control group is depicted.
DISCUSSION
Previous animal studies demonstrated that exposure to DDT increased the rate of lymphomas, as well as lung and hepatic benign and malignant tumors [9, 11]. Moreover, both in vitro and in vivo studies revealed that DDT has xenoestrogenic properties resulting in hormone-related pathologies [1, 33]. Controversial data have accumulated regarding the effects of DDT in humans; however, according to the International Agency for Research on Cancer, this compound is referred to as “possibly carcinogenic to humans”, and it may be applied only for prevention of malaria.
In 2012, Tilghman et al. reported that exposure to DDT results in altered expression of miR-1915, -923, -1308, -15b, -27b, -193a, -16, -149, -99b, and -342 [18]. Because DDT is an endocrine disruptor, to study a mechanism of its action we assessed the impact induced by DDT on microRNAs whose expression is usually disturbed during cancer in organs with high ER and PGR expression, such as uterus and ovaries, by specifically focusing on microRNA whose potential target genes involved in hormonal carcinogenesis. Our results demonstrated that a single DDT exposure resulted in significantly upregulated ovarian miR-429 and miR-205 level by 2- and 3-fold, respectively, as well as uterine miR-205 expression by 5-fold (Fig. 4). Chronically DDT-treated rats were found to have downregulated ovarian miR-222 as well as miR-126a and -205 by 2- and 3-fold, respectively, and upregulated uterine miR-221 expression by 3.5-fold (Figs. 5 and 6) after a high-dose DDT exposure. It is known that in humans these microRNAs regulate expression of crucial tumor suppressors and oncogenes such as JPH4 [20], PTEN [29, 30], CDKN1B [22], MYC [31], ZEB1, SIP1 [32], etc. In this light, our data suggest that DDT may exert carcinogenic effects by changing their expression. To confirm the effects elicited by DDT on microRNA expression, we measured mRNA levels for genes Cdkn1b, Pten, Dicer1, E2f1, Slc7a5, Itga6, and Bcl2 as potential targets for miR-221, -222, -429, -205, and -126a. We found that the level of mRNA for Pten and Dicer1 was significantly changed in DDT-treated rats. In particular, the relative level of mRNA of Pten was downregulated in ovaries and uterus by 2-fold after a single high-dose exposure to DDT (Fig. 7), matching to significantly upregulated miR-205 and -429 in these organs. These results agree with data showing that in endometrial cancer cells the level of mRNA Pten is often decreased, whereas for miR-205 it is elevated [30]. In addition, the level of uterine mRNA Dicer1 as a potential target for miR-221/222 was downregulated 2-fold after chronic exposure to high-dose DDT (Fig. 7), whereas in ovaries it tended to increase. Notably, in endometrial cancer cells downregulated expression of Dicer1 contributes to cancer progression [34]. Therefore, the data suggest that in the uterus DDT may exert carcinogenic effects.
We also examined effects of DDT on the level of mRNA for genes Esr1 and Cyp19a1, as well as ERα targets such as Ccnd1 and Pgr due to ability of DDT to affect activity of aromatase [16, 17], ER, and expression of its target genes, and for assessing the role of microRNAs-221, -222, -429, -205, and -126a in controlling key genes involved in hormonal carcinogenesis. We found that chronic exposure to DDT decreased the level of mRNA of Esr1, which was significant in ovaries after high-dose DDT treatment, albeit non-significant after a single-dose exposure (Figs. 1-3). These data fit to the earlier evidence that high-dose ligand exposure downregulates receptor expression as one of the cellular adaptation responses aimed at controlling cell sensitivity to various stimuli [35, 36]. On the other hand, xenoestrogenic effects of DDT were further confirmed by downregulated mRNA expression of the Cyp19a1 gene after both single and chronic DDT exposure. Because this gene codes for the enzyme catalyzing production of estrogens from androgens, its downregulated expression points at suppressed synthesis of endogenous estrogens, which may represent a response to an increased DDT-elicited enzymatic activity. In particular, both uterine and ovarian mRNA level for the Cyp19a1 gene was decreased by 2-fold after a single DDT exposure (Fig. 1), whereas in the uterus of chronic high-dose DDT-treated rats it was downmodulated by 2-fold (Fig. 3). Importantly, this gene is considered as a potential target for miR-221, -222, and -205, and expression of miR-205 turned out to be elevated after a single DDT exposure, whereas uterine miR-221 expression was upregulated after chronic high-dose DDT exposure. We demonstrated that Cyp19a1 mRNA level changed insignificantly only in ovaries, and that was paralleled with significantly downregulated miR-222 and miR-205 expression after chronic DDT exposure. These data suggest that miR-221, -222, and miR-205 might be involved in regulating Cyp19a1 expression. It should be emphasized that as in rats, the latter gene in humans is also considered as a potential target for miR-205-5p (Table 3), and expression of miR-205 is often downmodulated in breast cancer [37, 38]. This suggests that in estrogen-positive cancer downregulated expression of miR-205 may promote cancer progression via upregulating level of Cyp19a1 mRNA and subsequently increased estrogen production.
Expression of Ccnd1 targeted by ERα was elevated in the uterus both after single and chronic DDT exposure (by 2.6- and 2-fold, respectively). It is known that expression of this gene is upregulated via both receptors CAR and ERα activated by DDT [12, 14, 39]. Because in the uterus CAR is expressed at a low level unlike ERα, it is reasonable to assume that upregulated Ccnd1 expression after DDT exposure is mediated via ERα. In ovaries, Ccnd1 expression did not significantly change, perhaps being related to the lower level of ERα they express [40]. Another gene, Pgr, targeted by ERα was upregulated 2-fold in the uterus of rats chronically treated with high-dose DDT (Fig. 3). Perhaps in the uterus of rats treated with single dose DDT the lack of upregulated Pgr mRNA was related to the fact that this gene is considered as a potential target for miR-205, whose uterine level was upregulated 5-fold.
Thus, our study demonstrated that DDT exposure results in altered expression of oncogenic and tumor suppressor microRNAs such as miR-221, -222, -205, -126a, and -429 as well as their target genes necessary for regulating cell proliferation and translation (Pten, Dicer1) and the genes involved in hormonal carcinogenesis (Esr1, Pgr, Ccnd1, Cyp19a1). Therefore, expression of Pgr and Cyp19a1 seems to be also controlled by these microRNAs. Such changes in expression of microRNAs and mRNA level after exposure to DDT had dose-, tissue-, and time-dependent pattern (chronic or single exposure). These data suggest that the epigenetic effects triggered by DDT as a potential carcinogen may be based on at least two mechanisms: (i) activation of ERα followed by altered expression of the target genes encoding receptor Pgr and Ccnd1 as well as impaired expression of Cyp19a1, thereby, affecting cell hormone balance; and (ii) changed expression of microRNAs resulting in impaired expression of relevant target genes including key genes in hormonal carcinogenesis, e.g. downregulated expression of Cyp19a1 mRNA.
Acknowledgments
This study was performed with financial support from the Russian Science Foundation (project No. 15-15-30012).
REFERENCES
1.Jaga, K. (2000) What are the implications of the
interaction between DDT and estrogen receptors in the body? Med.
Hypotheses, 54, 18-25.
2.Yu, Y., Yang, O., Fazli, L., Rennie, P. S., Gleave,
M. E., and Dong, X. (2015) Progesterone receptor expression during
prostate cancer progression suggests a role of this receptor in stromal
cell differentiation, Prostate, 75, 1043-1050.
3.Tangen, I. L., Werner, H. M., Berg, A., Halle, M.
K., Kusonmano, K., Trovik, J., Hoivik, E. A., Mills, G. B., Krakstad,
C., and Salvesen, H. B. (2014) Loss of progesterone receptor links to
high proliferation and increases from primary to metastatic endometrial
cancer lesions, Eur. J. Cancer, 50, 3003-3010.
4.Halon, A., Materna, V., Drag-Zalesinska, M.,
Nowak-Markwitz, E., Gansukh, T., Donizy, P., Spaczynski, M., Zabel, M.,
Dietel, M., Lage, H., and Surowiak, P. (2011) Estrogen receptor alpha
expression in ovarian cancer predicts longer overall survival,
Pathol. Oncol. Res., 17, 511-518.
5.Shanle, E. K., and Xu, W. (2011) Endocrine
disrupting chemicals targeting estrogen receptor signaling:
identification and mechanisms of action, Chem. Res. Toxicol.,
24, 6-19.
6.Gao, H., Yang, B. J., Li, N., Feng, L. M., Shi, X.
Y., Zhao, W. H., and Liu, S. J. (2015) Bisphenol A and
hormone-associated cancers: current progress and perspectives,
Medicine (Baltimore), 94, e211.
7.Flor, S., He, X., Lehmler, H. J., and Ludewig, G.
(2016) Estrogenicity and androgenicity screening of PCB sulfate
monoesters in human breast cancer MCF-7 cells, Environ. Sci. Pollut.
Res. Int., 23, 2186-2200.
8.Ceballos-Laita, L., Calvo-Begueria, L., Lahoz, J.,
Bes, M. T., Fillat, M. F., and Peleato, M. L. (2015) γ-Lindane
increases microcystin synthesis in microcystis aeruginosa PCC7806,
Mar. Drugs, 13, 5666-5680.
9.Turusov, V., Rakitsky, V., and Tomatis, L. (2002)
Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and
risks, Environ. Health Perspect., 110, 125-128.
10.Longnecker, M. P., Rogan, W. J., and Lucier, G.
(1997) The human health effects of DDT
(dichlorodiphenyltrichloroethane) and PCBs (polychlorinated biphenyls)
and an overview of organochlorines in public health, Annu. Rev.
Public Health, 18, 211-244.
11.Turusov, V. S., Day, N. E., Tomatis, L., Gati,
E., and Charles, R. T. (1973) Tumors in CF-1 mice exposed for six
consecutive generations to DDT, J. Natl. Cancer Inst.,
51, 983-997.
12.Kiyosawa, N., Kwekel, J. C., Burgoon, L. D.,
Dere, E., Williams, K. J., Tashiro, C., Chittim, B., and Zacharewski,
T. R. (2008) Species-specific regulation of PXR/CAR/ER-target genes in
the mouse and rat liver elicited by o,p'-DDT, BMC Genomics,
9, 487.
13.Robison, A. K., Schmidt, W. A., and Stancel, G.
M. (1985) Estrogenic activity of DDT: estrogen-receptor profiles and
the responses of individual uterine cell types following o,p'-DDT
administration, J. Toxicol. Environ. Health, 16,
493-508.
14.Yarushkin, A. A., Kazakova, Yu. A., Demin, D. S.,
Pustyl’nyak, V. O., and Gulyaeva, L. F. (2012) Expression of
receptor CAR and ERα target genes in the liver of DDT-treated
mice, Vestnik NGU, 10, 5-13.
15.Alao, J. P. (2007) The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention, Mol. Cancer, 6, 24.
16.You, L., Sar, M., Bartolucci, E., Ploch, S., and
Whitt, M. (2001) Treatment of hepatic aromatase by p,p'-DDE in adult
male rats, Mol. Cell. Endocrinol., 178, 207-214.
17.Holloway, A. C., Stys, K. A., and Foster, W. G.
(2005) DDE-induced changes in aromatase activity in endometrial stromal
cells in culture, Endocrine, 27, 45-50.
18.Tilghman, S. L., Bratton, M. R., Segar, H. C.,
Martin, E. C., Rhodes, L. V., Li, M., McLachlan, J. A., Wiese, T. E.,
Nephew, K. P., and Burow, M. E. (2012) Endocrine disruptor regulation
of microRNA expression in breast carcinoma cells, PLoS One,
7, e32754.
19.Gulyaeva, L. F., Chanyshev, M. D., Kolmykov, S.
K., Ushakov, D. S., and Nechkin, S. S. (2016) Effect of xenobiotics on
microRNA expression in rat liver, Biomed. Khim., 62,
154-159.
20.Yanokura, M., Banno, K., Kobayashi, Y., Kisu, I.,
Ueki, A., Ono, A., Masuda, K., Nomura, H., Hirasawa, A., Susumu, N.,
and Aoki, D. (2010) MicroRNA and endometrial cancer: roles of small
RNAs in human tumors and clinical applications (review), Oncol.
Lett., 1, 935-940.
21.Valastyan, S., and Weinberg, R. A. (2011) Roles
for microRNAs in the regulation of cell adhesion molecules, J. Cell
Sci., 124, 999-1006.
22.Chegini, N. (2010) Uterine microRNA signature and
consequence of their dysregulation in uterine disorders, Anim.
Reprod., 7, 117-128.
23.Yang, J., Han, S., Huang, W., Chen, T., Liu, Y.,
Pan, S., and Li, S. (2014) A meta-analysis of microRNA expression in
liver cancer, PLoS One, 9, e114533.
24.Zhao, J. J., Lin, J., Yang, H., Kong, W., He, L.,
Ma, X., Coppola, D., and Cheng, J. Q. (2016) MicroRNA-221/222
negatively regulates estrogen receptor α and is associated with
tamoxifen resistance in breast cancer, J. Biol. Chem.,
291, 22859.
25.Cui, W., Li, Q., Feng, L., and Ding, W. (2011)
MiR-126-3p regulates progesterone receptors and involves development
and lactation of mouse mammary gland, Mol. Cell Biochem.,
355, 17-25.
26.Kinose, Y., Sawada, K., Nakamura, K., and Kimura,
T. (2014) The role of microRNAs in ovarian cancer, Biomed Res.
Int., 249393.
27.Wang, W., and Luo, Y. (2015) MicroRNAs in breast
cancer: oncogene and tumor suppressors with clinical potential, J.
Zhejiang Univ. Sci. B, 16, 18-31.
28.Yanokura, M., Banno, K., Iida, M., Irie, H.,
Umene, K., Masuda, K., Kobayashi, Y., Tominaga, E., and Aoki, D. (2015)
MicroRNAs in endometrial cancer: recent advances and potential clinical
applications, EXCLI J., 14, 190-198.
29.Suk, K. K., Peng, H., Tang, H., Cho, M. E., Peng,
J., Aller, M., and Yang, H. (2012) Recent advances of miRNA involvement
in hepatocellular carcinoma and cholangiocarcinoma, Open J. Intern.
Med., 2, 135-162.
30.Karaayvaz, M., Zhang, C., Liang, S., Shroyer, K.
R., and Ju, J. (2012) Prognostic significance of miR-205 in endometrial
cancer, PLoS One, 7, e35158.
31.Huang, X., Yao, J., Huang, H., Wang, C., Ma, Y.,
Xia, Q., and Long, X. (2013) MicroRNA-429 modulates hepatocellular
carcinoma prognosis and tumorigenesis, Gastroenterol. Res.
Pract., 804128.
32.Niu, K., Shen, W., Zhang, Y., Zhao, Y., and Lu,
Y. (2015) MiR-205 promotes motility of ovarian cancer cells via
targeting ZEB1, Gene, 574, 330-336.
33.Aube, M., Larochelle, C., and Ayotte, P. (2008)
1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p'-DDE) disrupts
the estrogen–androgen balance regulating the growth of
hormone-dependent breast cancer cells, Breast Cancer Res.,
10, 16.
34.Wang, X. J., Jiang, F. Z., Tong, H., Ke, J. Q.,
Li, Y. R., Zhang, H. L., Yan, X. F., Wang, F. Y., and Wan, X. P. (2017)
Dicer1 dysfunction promotes stemness and aggression in endometrial
carcinoma, Tumor Biol., 39, doi:
10.1177/1010428317695967.
35.Ellison-Zelski, S. J., Solodin, N. M., and
Alarid, E. T. (2009) Repression of ESR1 through actions of estrogen
receptor alpha and Sin3A at the proximal promoter, Mol. Cell
Biol., 29, 4949-4958.
36.Stoica, G. E., Franke, T. F., Moroni, M.,
Mueller, S., Morgan, E., Iann, M. C., Winder, A. D., Reiter, R.,
Wellstein, A., Martin, M. B., and Stoica, A. (2003) Effect of estradiol
on estrogen receptor-alpha gene expression and activity can be
modulated by the ErbB2/PI 3-K/Akt pathway, Oncogene, 22,
7998-8011.
37.Iorio, M. V., Casalini, P., Piovan, C., Di Leva,
G., Merlo, A., Triulzi, T., Menard, S., Croce, C. M., and Tagliabue, E.
(2009) microRNA-205 regulates HER3 in human breast cancer, Cancer
Res., 69, 2195-2200.
38.Wu, H., Zhu, S., and Mo, Y. Y. (2009) Suppression
of cell growth and invasion by miR-205 in breast cancer, Cell
Res., 19, 439-448.
39.Griekspoor, A., Zwart, W., Neefjes, J., and
Michalides, R. (2007) Visualizing the action of steroid hormone
receptors in living cells, Nucl. Recept. Signal., 5,
e003.
40.Zin, S. R., Omar, S. Z., Khan, N. L., Musameh, N.
I., Das, S., and Kassim, N. M. (2013) Effects of the phytoestrogen
genistein on the development of the reproductive system of
Sprague–Dawley rats, Clinics (Sao Paulo), 68,
253-262.