Mitochondria-Targeted Antioxidant SkQ1 (10-(6´-Plastoquinonyl)decyltriphenylphosphonium Bromide) Inhibits Mast Cell Degranulation in vivo and in vitro
M. A. Chelombitko1*, O. A. Averina2, T. V. Vasilyeva1, O. Yu. Pletiushkina3, E. N. Popova3, A. V. Fedorov1, B. V. Chernyak3, V. S. Shishkina1, and O. P. Ilinskaya1
1Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: atma69@yandex.ru2Lomonosov Moscow State University, Institute of Mitoengineering, 119992 Moscow, Russia
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received August 15, 2017; Revision received September 17, 2017
The therapeutic effect of mitochondria-targeted antioxidant 10-(6´-plastoquinonyl)decyltriphenylphosphonium bromide (SkQ1) in experimental models of acute inflammation and wound repair has been shown earlier. It was suggested that the antiinflammatory activity of SkQ1 is related to its ability to suppress inflammatory activation of the vascular endothelium and neutrophil migration into tissues. Here, we demonstrated that SkQ1 inhibits activation of mast cells (MCs) followed by their degranulation and histamine release in vivo and in vitro. Intraperitoneal injections of SkQ1 in the mouse air-pouch model reduced the number of leukocytes in the air-pouch cavity and significantly decreased the histamine content in it, as well as suppressing MC degranulation in the air-pouch tissue. The direct effect of SkQ1 on MCs was studied in vitro in the rat basophilic leukemia RBL-2H3 cell line. SkQ1 inhibited induced degranulation of RBL-2H3 cells. These results suggest that mitochondrial reactive oxygen species are involved in the activation of MCs. It is known that MCs play a crucial role in regulation of vascular permeability by secreting histamine. Suppression of MC degranulation by SkQ1 might be a significant factor in the antiinflammatory activity of this mitochondria-targeted antioxidant.
KEY WORDS: inflammation, mast cell, degranulation, histamine, mitochondria-targeted antioxidantDOI: 10.1134/S0006297917120082
Abbreviations: anti-DNP IgE, mouse monoclonal IgE antibodies against dinitrophenol; DNP-BSA, DNP-conjugated bovine serum albumin; MC, mast cell; mtROS, mitochondrial ROS; PMA, phorbol myristate acetate (phorbol ester); RBL-2H3, rat basophilic leukemia cell line; ROS, reactive oxygen species; SkQ1, 10-(6´-plastoquinonyl)decyltriphenylphosphonium bromide; TGF-β1, transforming growth factor β1; TNF, tumor necrosis factor.
Mast cells (MCs) are one of the most important cell populations in
connective tissue. They maintain local tissue homeostasis and
participate in multiple processes, such as regulation of vascular
permeability and blood clotting, innate and adaptive immunity, and
allergic response [1-3]. The
properties of MCs are determined by a broad array of biologically
active compounds that are stored in the MC intracellular granules and
released following MC activation. MCs are key initiators and regulators
of inflammation; they secrete inflammatory mediator molecules, the most
important of which is histamine. Histamine increases vascular
permeability and causes edema of surrounding tissues, thereby promoting
migration of leukocytes to a site of inflammation [4-6].
Recent studies have demonstrated that reactive oxygen species (ROS) play and important role in various aspects of MC physiology [7-10]. The release of inflammatory mediators from MC granules induced by chemical agents and physiological stimuli is accompanied by ROS generation [10, 11]. However, the sources of ROS and the involvement of ROS in intracellular signaling in MCs are still poorly studied [10]. Mitochondria are one of the sources of ROS. The role of mitochondrial ROS (mtROS) in intracellular signaling has been studied using mitochondria-targeted antioxidants that are selectively accumulated in mitochondria due to the presence of membranophilic cations. One of the best-studied mitochondria-targeted antioxidants is 10-(6´-plastoquinonyl)decyltriphenylphosphonium bromide (SkQ1) [12].
SkQ1 exhibits therapeutic effect in many disorders associated with chronic or acute inflammation [13-21]. Thus, SkQ1 prevents death of mice after intravenous injection of a lethal dose of tumor necrosis factor (TNF) [22]. This model partially reproduces systemic the inflammatory response syndrome that is one of the major causes of death of intensive care patients [23]. SkQ1 accelerates the healing of full-thickness dermal wounds in aged and diabetic mice mostly via accelerated resolution of the inflammatory phase of wound healing [16, 19]. The SkQ1 analog SkQR1, that differs from SkQ1 in its cation fragment, prevents development of pyelonephritis in mice [15] and displays therapeutic effect after ischemia/reperfusion in kidneys and brain [24, 25]. Long-term application of SkQ1 sufficiently suppresses inflammatory activation (expression of adhesion molecules) of endothelium in aortas of old mice [20], which indicates an important role of mtROS in chronic inflammation. SkQ1 downregulates the TNF-induced expression of E-selectin and adhesion molecules ICAM-1 and VCAM-1 in cultured endothelial cells, thereby inhibiting adhesion of neutrophils to the endothelial cell monolayer [20]. In the same experimental model, SkQ1 decreased secretion of proinflammatory cytokines IL-6 and IL-8 [20] and prevented deterioration of cell–cell contacts [21, 22, 26] and apoptosis [27]. It was suggested that the excessive activation antiinflammatory activity of SkQ1 is realized through suppression of the vascular epithelium. Also, SkQ1 downregulates activation of neutrophils by the N-formyl-methionyl-leucyl-phenylalanine (fMLP) peptide typical for bacterial cell wall proteins. The mtROS are involved in degranulation of neutrophils and activation of NADPH oxidase, which serves as the main source of ROS and determines the antibacterial activity of these cells [28].
To study the anti-inflammatory activity of SkQ1, we used a model of carrageenan-induced inflammation in the subcutaneous air-pouch in mice. In this model, leukocyte mobilization to the inflammation site is predominantly caused by cytokines produced by macrophages, fibroblasts, and MCs residing in the air-pouch wall [29]. Carrageenan increases the number of MCs in the air-pouch tissues and activates their degranulation [30]. Inflammation induction by carrageenan in the MC-deficient WBC6F1/J-Sl/Sl(d) mice results in a considerable (40%) decrease in the number of leukocytes in the exudate [31]. Using this model, we showed that SkQ1 significantly decreased the number of cells in the exudate [21]. In this work, we studied the effect of SkQ1 on the content and status of MCs in the air-pouch walls and on the concentration of histamine and the number of cells in the air-pouch lavage fluid in the absence of proinflammatory stimulation by carrageenan. The direct effect of SkQ1 on MCs was studied in vitro in the rat basophilic leukemia RBL-2H3 cell line.
MATERIALS AND METHODS
Male mice (F1 hybrids of CBA × C57Bl/6 crossing) used in in vivo experiments were kindly provided by the Animal Facility of the Institute of Mitoengineering, Lomonosov Moscow State University. All procedures were done in accordance with Directive 2010/63/EU of the European Parliament and the Federation of European Laboratory Animal Science Associations (FELASA). When necessary, the animals were anesthetized with Zoletil (40 mg/kg body weight; Virbac).
SkQ1 (10-(6´-plastoquinonyl)decyltriphenylphosphonium bromide) was kindly provided by the Institute of Mitoengineering, Lomonosov Moscow State University.
Effect of intraperitoneally injected SkQ1 on the MC population in subcutaneous air-pouch model. All experiments were performed in 18-month-old mice. To create the air pouch, 4 ml of sterile air was injected subcutaneously between the scapulae; the injection was repeated with 2 ml of air 4 days later [32]. Control mice (n = 5) were then injected intraperitoneally with 0.9% NaCl (physiological saline); experimental animals were injected intraperitoneally with SkQ1 solution in physiological saline (250 nmol/kg body weight). These injections (5 ml/kg body weight) were performed daily for 7 days starting from the time of air-pouch formation. On day 7, 2 ml of phosphate buffered saline (PBS) containing 5.4 mM EDTA was injected into the air pouch, and the content of the pouch was aspirated. The volume of the aspirated fluid was measured, and the number of cells in it was determined using a cell-counting chamber. To estimate the intracellular histamine content, the aspirate was centrifuged for 5 min at 1100g at 4°C, and the pellet was lysed by adding 250 µl of 0.1% Triton X-100 in PBS and incubating for 15 min at 37°C. The lysates were centrifuged at 4390g for 7 min at 4°C, and the histamine content in the supernatant was determined.
The content of the air-pouch cavity was analyzed for the presence of MCs. MCs were identified in dried smears after fixation with 10% formalin and staining with 0.1% toluidine blue by the presence of specific metachromatically-stained granules and counted (out of 1000 cells analyzed) under a Leica DM1000 microscope.
The histamine content in the supernatant and cell lysate was determined by reaction with orthophthalic aldehyde according to a standard method [33]. Aliquots (100 µl) of analyzed samples were placed into wells of a 96-well plate and mixed with 20 µl of 1 M NaOH and then with 5 µl of 1% orthophthalic aldehyde (Sigma, USA). The formed fluorophore was stabilized with 3 M HCl, and its fluorescence was measured at 460 nm with a Thermo Fluoroskan Ascent spectrofluorometer (λex = 355 nm).
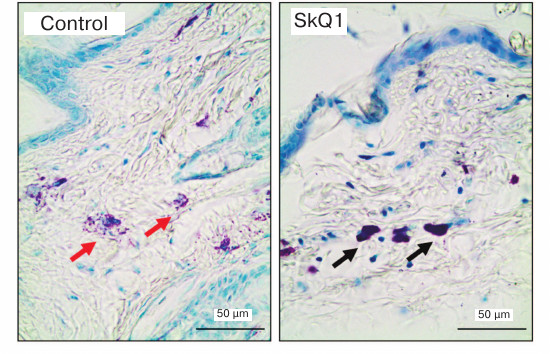
For histological analysis, the wall of the air pouch was cut out immediately after aspiration, fixed in 10% formalin in PBS for 24 h, subjected to routine histological treatment, and embedded in paraffin. Histological sections (4-µm thick) were stained with 0.1% toluidine blue and assayed for the presence of MCs. MCs were counted in the outer (upper) wall of the air pouch in 20 fields of vision (10 in the dermis and 10 in the subdermal layer) using a ×40 objective. To estimate the functional state of the MC population, two groups of cells were analyzed: “dark” cells densely populated with metachromatic granules that were poorly distinguishable from each other, and “light” cells with sparsely located and clearly distinguishable granules (Fig. 1). The ratio between these two types of cells was determined by analyzing 100 cells in total using a ×100 objective. The percentage of degranulating MCs surrounded by the metachromatic granules was also estimated.
Fig. 1. Histological sections of air-pouch wall in control (physiological saline) and experimental (SkQ1) mice. Toluidine-blue staining; black and red arrows, MCs with dense and sparse metachromatic granules, respectively.
Effect of intraperitoneally injected SkQ1 on population of peritoneal MCs. All experiments were performed in 16-week-old mice (n = 22).
In the first group of experiments, experimental mice (n = 4) were injected intraperitoneally with SkQ1 in physiological saline (250 nmol/kg body weight); control mice (n = 3) were injected with physiological saline (5 ml/kg body weight). Six hours after injection, peritoneal cells were isolated from both groups of animals.
In the second group of experiments, mice from the experimental group (n = 8) received daily intraperitoneal injection of SkQ1 in physiological saline (250 nmol/kg body weight) for 7 days; animal of the control group (n = 7) were injected with physiological saline (5 ml/kg body weight). On day 8, the animals were euthanized, and 5 ml of PBS containing 5.4 mM EDTA was injected intraperitoneally. After massaging the abdominal wall, the abdominal cavity was open and peritoneal fluid was collected. The volume of the peritoneal fluid was determined, and the cells in it were counted using a cell-counting chamber. Smears of the peritoneal fluid were prepared, and the number of MCs was counted on slides after their fixation with 10% formalin and staining with 0.1% toluidine blue.
Histamine content in the liquid portion of the peritoneal lavage fluid and cell lysates was determined by reaction with orthophthalic aldehyde as described above.
Effect of SkQ1 on degranulation of RBL-2H3 cells. The effect of SkQ1 on functional activity of MCs was investigated by assaying its ability to induce MC degranulation. As a model of MCs, we used the RBL-2H3 cell line (kindly provided by E. V. Kiseleva, Laboratory of Cell Biology, Koltsov Institute of Developmental Biology, Russian Academy of Sciences), which is close to MCs in its functional properties. This commercially available cell line was derived from the peripheral blood of Wistar rats with chemically induced basophilic leukemia and is widely used as an experimental model in studies of activation mechanisms in MCs and basophils [34].
RBL-2H3 cells were grown in cell culture flasks in α-MEM medium supplemented with 2 mM L-glutamine and 10% heat-inactivated fetal bovine serum (HI-FBS) (PanEko, Russia). The cells were passaged every 3 days at 1 : 4 to 1 : 8 dilution. For experiments, the cells were plated in 24- and 48-well plates at 100,000 cell/ml. After cell attachment to the well, SkQ1 was added to the culturing medium at final concentrations of 0.2, 2, 20, 200, or 400 nM, and the cells were grown in an incubator for 4 days at 37°C in 5% CO2. MC degranulation was induced by two different methods. In the first case, 50 nM phorbol ester (PMA; Sigma) and 1 µM calcium ionophore A23187 (Sigma) were added to the culturing medium on day 5, and the cells were cultured for another 24 h. In the second case, the cells were sensitized on day 5 with 0.4 µg/ml anti-DNP IgE (mouse anti-dinitrophenol IgE antibodies; Sigma) for 16 h. The antibodies were then washed off, and the cells were activated with 0.1 µg/ml DNP-BSA (DNP-conjugated bovine serum albumin; Molecular Probes, USA) in Tyrode buffer (135 mM NaCl, 5 mM KCl, 20 mM HEPES, 1.8 mM CaCl2, 1 mM MgCl2, 6 mM glucose, 1 mg/ml BSA, pH 7.4) for 18 min [35]. Four hours before conducting the experiment, the culturing medium was replaced with serum-free medium (in the case of PMA and A23187 stimulation) or fresh medium (in the case of cell sensitization with anti-DNP IgE followed by stimulation with DNP-BSA).
The extent of degranulation of the activated RBL-2H3 cells was estimated from the activity of β-hexosaminidase in the conditioned medium or in the cell lysate by the release of p-nitrophenol from the substrate 4-nitrophenyl N-acetyl-β-D-glucosaminide (Sigma) by a standard method [36]. After activation of degranulation, the culturing medium was aspirated. The cells were lysed with an equal volume of 0.1% Triton X-100 in the serum-free medium or Tyrode buffer and incubated for 15 min at 37°C. The conditioned medium was centrifuged for 5 min at 4390g at 4°C. The supernatant was assayed for β-hexosaminidase activity after adding substrate. The reaction was stopped in 2 h with 0.2 M glycine-NaOH buffer, and absorbance of the samples was measured with an iMARK Microplate Reader at 410 nm. The relative content (%) of β-hexosaminidase in the samples was determined using the formula A/(A + B) × 100%, where A is optical density of the conditioned medium, and B is optical density of the cell lysate.
Cytotoxic activity of SkQ1 was estimated in the MTT test. RBL-2H3 cells were plated in 96-well plates at 100,000 cells/ml medium. After the cells attached to the plate, SkQ1 was added to the culturing medium in at concentrations from 0.02 to 2000 nM, and the cells were incubated at 37°C under 5% CO2 for another 4 days. On day 5, 20 µl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Chem Impex Intl Inc, USA)) was added to 5 mg/ml, and the cells were incubated for 3 h at 37°C under 5% CO2. The precipitate was dissolved in 100 µl DMSO. The absorbance of the samples was determined with the iMARK Microplate Reader at 540 nm.
The data were analyzed statistically with the STATISTICA 8.0 program package; graphs were plotted with the GraphPad Prism6 program. The significance of differences between groups was estimated using the two-tailed nonparametric Mann–Whitney test; differences at p < 0.05 were considered significant; differences at p < 0.1 were considered trends. Data in all graphs are presented as median with upper and lower quartiles (in several cases, as the interquartile range) or as maximal and minimal values in the sample (not shown in some cases).
RESULTS
Effect of intraperitoneal injections of SkQ1 on the MC population in the air-pouch model. As shown earlier, MCs play a key role in formation of inflammatory exudate in the air-pouch model of carrageenan-induced inflammation in mice [21]. The structure of the pouch wall greatly determines the character of the inflammatory response to the irritant [29]. Earlier, we found that SkQ1 significantly decreases the number of cells in the inflammatory exudate from the subcutaneous air pouch after carrageenan-induced acute inflammation in mice [21]. Based on these observations, we suggested that one of the mechanisms of the antiinflammatory activity of SkQ1 might be its action on the MCs in the air-pouch wall. To test this hypothesis, we investigated the effect of SkQ1 on activation of MCs in the air-pouch wall in the absence of carrageenan stimulation.
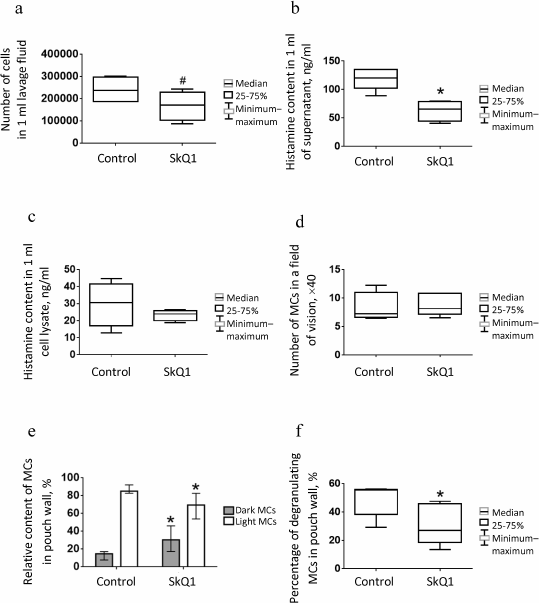
Studying lavage fluid from subcutaneous air pouch in mice injected with SkQ1 in comparison to the control animals revealed a trend for decrease (1.4-fold, p = 0.08) in absolute number of cells in this fluid (Fig. 2a). Morphological analysis of the exudate showed the presence of neutrophils, lymphocytes, monocytes/macrophages, and an insignificant number of erythrocytes and platelets. No MCs were found in samples from either control or experimental groups.
Fig. 2. MC content and histamine levels in lavage fluid from subcutaneous air pouch and estimation of MC population state in the air-pouch wall in control (physiological saline, n = 5) and experimental (SkQ1, n = 5) mice: a) absolute number of MCs in 1 ml of lavage fluid; b) histamine content in 1 ml of lavage fluid; c) histamine content in cell lysate. MCs in the air-pouch wall: d) total number of MCs; e) percentage content of “dark” and “light” MCs; f) percentage content of degranulating MCs. Asterisk (*), significant difference from control group (p < 0.05); #, trend to difference (p < 0.1). Data in Fig. 2e are shown as median and interquartile range.
The histamine content in supernatant of the air-pouch lavage fluid was two times lower in the experimental group (p = 0.02) than in the control animals (Fig. 2b), which might be related to suppression of MC degranulation in the pouch wall (see below). Histamine was also present, although in insignificant amounts, in the cell lysates (Fig. 2c) due, probably, to the ability of macrophages and neutrophils to phagocytize histamine-containing granules from MCs [37] or to histamine-synthesizing activity of platelets [38]. The content of histamine in cell lysates from the control and experimental groups was the same (Fig. 2c).
Counting MCs in histological sections of the pouch wall revealed no significant difference between control and experimental groups of mice (Fig. 2d); however, the number of “dark” MCs densely populated with poorly distinguishable granules was considerably higher, and therefore the number of “light” MCs with sparse well-distinguishable granules was lower in the experimental group (p = 0.01) (Fig. 2e). The number of degranulating cells surrounded by metachromatic granules was two times lower in the experimental mice than in the control animals (p = 0.04) (Fig. 2f).
These data show that daily intraperitoneal injection of SkQ1 at dose 250 nmol/kg body weight for 7 days did not affect the number of MCs in the air-pouch wall, but it considerably suppressed degranulation of these cells, thereby decreasing the levels of histamine and the number of leukocytes in the air-pouch cavity.
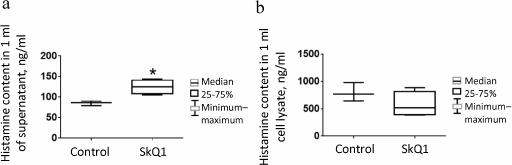
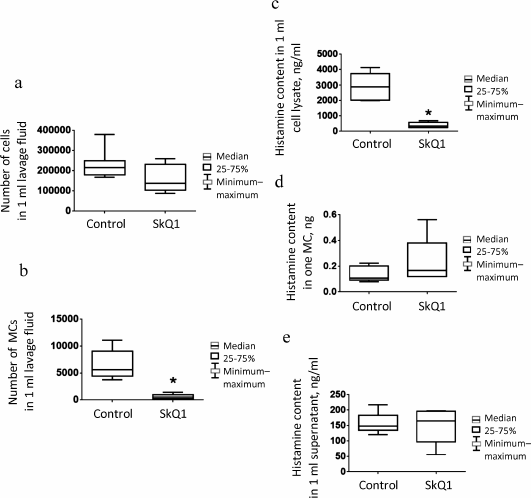
Effect of intraperitoneal injection of SkQ1 on population of peritoneal MCs. In the above-described air-pouch model, as well as in some other in vivo models [15, 21, 22], SkQ1 is injected intraperitoneally at dose 250 nmol/kg body weight, so that considering the amount of injected SkQ1 (10 nmol), volume of injected solution (200 µl), and average volume of the peritoneal fluid (~20 µl), the calculated concentration of injected SkQ1 at the time of injection is ~44 µM [39]. Six hours after SkQ1 injection, the histamine levels in the peritoneal fluid increased slightly (Fig. 3a), probably due to MC degranulation or MC death resulting from relatively high local dose of the antioxidant. No significant decrease in histamine content in cell lysates from control mice was found, presumably because of insufficient number of animals tested (Fig. 3b). The number of mature MCs (with metachromatic granules) in the peritoneal cavity of animals injected daily for 7 days with SkQ1 significantly decreased (p = 0.006) (Fig. 4b). The histamine levels in peritoneal cell lysates also decreased (p = 0.002) (Fig. 4c). However, no differences in the histamine content per MC (Fig. 4d), as well as in histamine levels in the peritoneal lavage fluid supernatants (Fig. 4e) were observed.
Fig. 3. Histamine content in peritoneal lavage fluid in control (n = 3) and experimental (n = 4) mice 6 h after single intraperitoneal injection of SkQ1 (experimental animals) and physiological saline (control animals): a) in 1 ml of supernatant; b) in peritoneal cell lysate. Asterisk, significant difference with control group (p < 0.05).
Fig. 4. Absolute number of cells, MC content, and histamine levels in peritoneal lavage fluid in control (physiological saline; n = 7) and experimental (SkQ1; n = 8) mice on day 8 after seven days of daily intraperitoneal injections: a) absolute number of cells in 1 ml of peritoneal lavage fluid; b) absolute number of MCs in 1 ml of peritoneal lavage fluid; c) histamine content in peritoneal cell lysate; d) average histamine content in one MC; e) histamine content in 1 ml of supernatant. Asterisk, significant difference with control group (p < 0.05).
The population of peritoneal fluid cells in animals, including mice, is characterized by the presence of a small number of MCs and leukocytes [40]. In our experiments, MCs comprised on average 2.3% of the total cell population; hence, the decrease in their content in experimental animals only slightly affected total number of cells in the peritoneal lavage fluid (Fig. 4a).
Therefore, intraperitoneal injection of SkQ1 in high concentrations did not significantly decrease total number of cells in peritoneal fluid but selectively affected the subpopulation of MCs.
Effect of SkQ1 on degranulation of RBL-2H3 cells. To determine if the observed suppression of MC degranulation in vivo might result from direct action of SkQ1 on these cells, we studied the effect of SkQ1 on spontaneous and induced degranulation of rat basophilic leukemia RBL-2H3 cells. This cell line is commonly used as a model for studying MC activation. Signaling mechanisms that regulate degranulation of RBL-2H3 cells and content of their secretory granules are almost identical to those in primary MCs and basophils [41]. β-Hexosaminidase, an enzyme that is used as a degranulation marker, is present in the same secretory granules as histamine.
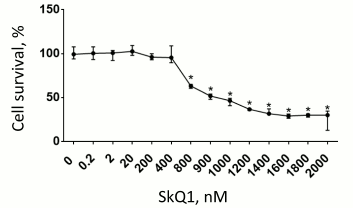
First, we estimated cytotoxic effect of various SkQ1 concentrations on RBL-2H3 cells and found that SkQ1 at concentrations of 800 nM and higher considerably decreases cell survival (Fig. 5). Therefore, the concentrations of SkQ1 that were used in the experiments on the influence of SkQ1 on RBL-2H3 cell degranulation were within the range that produced no cytotoxic effect on the cells.
Fig. 5. Cytotoxic effect of various SkQ1 concentrations (n = 3). Asterisk, significant difference from control group (p < 0.05). Data are shown as median and interquartile range.
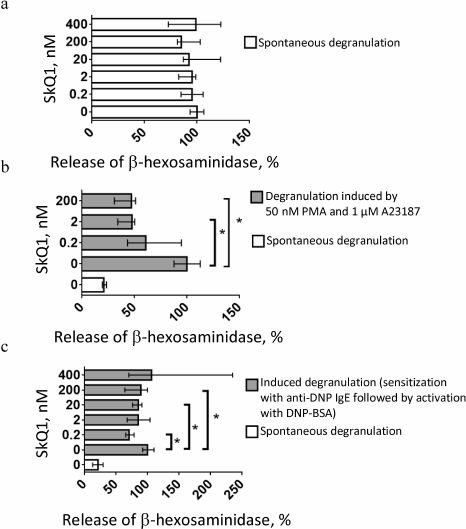
SkQ1 at concentrations from 0.2 to 400 nM produced no visible effect on spontaneous degranulation of RBL-2H3 cells (Fig. 6a). However, when degranulation was induced by 1 µM calcium ionophore A23187 in combination with 50 nM phorbol ester (PMA), SkQ1 at concentrations of 2 or 200 nM suppressed degranulation 2-fold (Fig. 6b). SkQ1 (0.2, 20, and 200 nM) considerably (by 20-30%) inhibited antigen (DNP-BSA)-stimulated degranulation of cells sensitized with anti-DNP IgE (Fig. 6c).
Fig. 6. Effect of SkQ1 concentration on (a) spontaneous and (b, c) induced degranulation of RBL-2H3 cells (n = 3) in the presence of (b) 50 nM PMA and 1 µM A23187 for 24 h or (c) 0.4 µg/ml anti-DNP IgE for 16 h and 0.1 µg/ml DNP-BSA for 18 min. Asterisks, significant difference between degranulation levels (p < 0.05). Data are shown as median and interquartile range.
DISCUSSION
Studying the antiinflammatory action of the mitochondria-targeted antioxidant SkQ1 in the air-pouch model in mice showed that intraperitoneal injection of SkQ1 causes a trend toward decrease in total number of cells (neutrophils, lymphocytes, and monocytes/macrophages) in the air pouch cavity (Fig. 2a). These results correlate well with our earlier observations that SkQ1 suppresses acute inflammation induced by introduction of carrageenan into the air pouch [21]. We found that SkQ1 does not affect the number of MCs but decreases the extent of their degranulation and histamine concentration in the exudate (Fig. 2b). Together with published data on the important role of MCs in the development of carrageenan-dependent inflammation in the air pouch model [30, 31], the results of our experiments suggest that the effect of SkQ1 on MC activation might represent of one of the mechanisms of antiinflammatory activity of SkQ1.
According to data accumulated so far, intraperitoneal injections of SkQ1 and its analogs produce significant therapeutic effect in models of various pathological conditions related to acute and chronic inflammation [14-25]. The dose and manner of SkQ1 application in our experiments were chosen based on data of previous studies [15, 21, 22]. However, it is possible that intraperitoneal injection creates high concentrations of SkQ1 in the peritoneal cavity, which might affect the state of cells that populate the peritoneal cavity and the peritoneum. We found that SkQ1 injection only slightly changes total number of cells in the peritoneal cavity but considerably decreases the content of mature MCs in it (Fig. 4b). Perhaps MCs are much more sensitive to the possible cell toxicity of SkQ1 than leukocytes.
We also observed increase in histamine concentration in peritoneal lavage fluid 6 h after intraperitoneal injection of SkQ1 (Fig. 3a), as well as decrease in content of mature peritoneal MCs after a week of daily intraperitoneal injections of SkQ1 (Fig. 4b). This might indicate that SkQ1 intraperitoneal injections at dose 250 nmol/kg body weight cause degranulation of peritoneal MCs and/or their death, which eventually leads to depletion of the pool of mature MCs. The absence of mature (i.e. able to degranulate) peritoneal MCs in our studies might be beneficial in the case of intraperitoneal inflammation because of leukocyte infiltration suppression [42] and decrease in levels of proinflammatory cytokines and chemokines, especially TNF [43]. The absence of peritoneal MCs was also found to promote survival in a model of bacterial sepsis in mice [44].
However, it should be noted that the decrease in content of peritoneal MCs could not explain the antiinflammatory properties of SkQ1 in various models not related to the initiation of inflammatory processes in the peritoneal cavity. In several studies, SkQ1 suppressed infiltration of neutrophils into a wound when applied orally for a long-term period [16, 19], as intraperitoneal injections [14], or in the content of wound coverings (data not shown). These methods of application do not create high local concentrations of the antioxidant in the peritoneal cavity, and therefore they do not affect the number of peritoneal MCs. Despite this and the absence of visible negative side effects of the intraperitoneal injections of SkQ1 and its analogs, the ability of SkQ1 to decrease the content of mature peritoneal MCs (and probably some other cell subpopulations in peritoneal fluid) should be considered when choosing the mode of antioxidant application.
The effects of intraperitoneal injection of SkQ1 on the population of peritoneal MCs might be related to its influence on survival of these cells. This hypothesis is corroborated by results of our in vitro studies, which demonstrated that SkQ1 concentrations exceeding 800 nM exhibit cytotoxic activity toward RBL-2H3 cells, as well as by earlier published data on the important role of ROS in MC survival [7-9]. SkQ1 might also modulate production of regulatory factors required for MC survival by cells of peritoneal exudate and peritoneum. Thus, fibroblast-produced TGF-β1 decreases MC survival in vivo [45]. At the same time, SkQ1 promotes biosynthesis of active TGF-β1 by fibroblasts in vitro [13]. Our results might also be explained by stimulation of peritoneal MC degranulation by high doses of SkQ1. This suggestion correlates well with data on possible prooxidative activity of high SkQ1 concentrations [12] and observations that induced oxidative stress can cause MC degranulation [46]. Perhaps intraperitoneally injected SkQ1 directly affects the cells that migrate into the peritoneal cavity from blood. Thus, after rat peritoneal cells had been lysed with distilled water, a small number of immature MC precursors containing no metachromatic granules appeared in the peritoneal cavity two days after the lysis. More mature granule-containing MCs were observed on day 6, while complete restoration of the mature MC populations happened only 10 days after the lysis [47] or even later [44]. This gives us reason to believe that SkQ1 can regulate migration of peritoneal fluid cells, especially in MCs. However, this hypothesis should be tested in further experiments on the dynamics of MC population restoration after treatment with SkQ1.
To study the direct effect of SkQ1 on MCs, we used the rat basophilic leukemia cell line RBL-2H3. SkQ1 at concentrations from 0.2 to 400 nM produced no effect on spontaneous degranulation of RBL-2H3 cells (Fig. 6a), but it inhibited degranulation stimulated by combined application of calcium ionophore A23187 and phorbol ester (PMA) (Fig. 6b). SkQ1 within a broad range of concentrations also suppressed degranulation of cells sensitized with anti-DNP IgE and then activated with DNP-BSA (Fig. 6c). Increase in SkQ1 concentration over 800 nM decreased RBL-2H3 cell survival (Fig. 5), which correlated with data on high sensitivity of MCs to toxic action of SkQ1 in the peritoneal cavity (Fig. 5b).
MC degranulation is accompanied by ROS generation; however, data on sources and role of ROS in MC activation are contradictory [10, 48]. Our results showed that low SkQ1 concentrations inhibit MC degranulation in vivo and in vitro, which correlates with earlier published data on suppression of MC degranulation in the presence of various ROS inhibitors and antioxidants [49-53]. The role of mtROS in activation of MCs is of great interest. Thus, thapsigargin (a compound that stimulates MC degranulation) induces an increase in ROS intracellular content directly via production of superoxide anion O2•¯ in mitochondria. mtROS generation is closely associated with Ca2+ entry into mitochondria [11]. Moreover, mtROS affect histamine synthesis in MCs. For example, MCs from bone marrow of Ucp2-deficient mice (Usp2 is a mitochondrial inner membrane protein that regulates mtROS production) contain elevated levels of histamine, which could be lowered by the superoxide dismutase mimetic MnTBAP preferentially accumulated in mitochondria [54]. It should be noted that MC degranulation is accompanied by translocation of mitochondria to the plasma membrane and by their fragmentation mediated by the antioxidant protein Drp1 [55]. mtROS generation might also be related to fragmentation of mitochondria [56, 57]. Besides, mitochondria-produced ROS, as well as ROS produced in other cell compartments, might directly affect the FcεRI signaling pathway that plays an important role in MC activation [10]. Therefore, mtROS might be involved in the MC activation through regulating histamine synthesis, mitochondrial concentration of Ca2+, translocation and fragmentation of mitochondria, and FcεRI signaling.
Based on the existing data, we can conclude that mtROS play an important role in MC activation that leads to MC degranulation and histamine release. Because histamine increases vascular permeability and promotes leukocyte migration to an area of inflammation, suppression of MC activation by the mitochondria-targeted antioxidant SkQ1 might contribute to the antiinflammatory activity of SkQ1. This effect, as well as earlier described suppression of inflammatory activation of endothelial cells [20, 22, 26, 27] and neutrophils [28], might underlie the therapeutic effect of SkQ1 and its analogs in models of inflammation-associated pathologies.
Acknowledgments
This work was supported by the Russian Science Foundation (project No. 14-50-00029, in vivo experiments) and by the Russian Foundation for Basic Research (project No. 16-04-01074, in vitro experiments).
REFERENCES
1.Omelyanenko, N. P., and Slutskiy, L. I. (2009)
Connective Tissue (Histology and Biochemistry) [in Russian],
Vol. 1, Izvestiya, Moscow.
2.Yarilin, A. A. (2010) Immunology [in
Russian], GEOTAR-Media, Moscow.
3.Da Silva, E., Jamur, M., and Oliver, C. (2014) Mast
cell function: a new vision of an old cell, J. Histochem.
Cytochem., 62, 698-738.
4.Stankiewicz, E., Wypasek, E., and Plytycz, B.
(2001) Short communication opposite effects of mast cell degranulation
by compound 48/80 on peritoneal inflammation in Swiss and CBA mice,
J. Pharmacol., 53, 149-155.
5.Kolaczkowska, E., Arnold, B., and Plytycz, B.
(2008) Mast cell involvement in zymosan-induced peritonitis in C57Bl/6
mice, Centr. Eur. J. Immunol., 33, 91-97.
6.Theoharides, T. C., Alysandratos, K. D., Angelidou,
A., Delivanis, D. A., Sismanopoulos, N., Zhang, B., and Kalogeromitros,
D. (2012) Mast cells and inflammation, Biochim. Biophys. Acta,
1822, 21-33.
7.Sly, L. M., Kalesnikoff, J., Lam, V., Wong, D.,
Song, C., Omeis, S., Chan, K., Lee, C. W., Siraganian, R. P., Rivera,
J., and Krystal, G. (2008) IgE-induced mast cell survival requires the
prolonged generation of reactive oxygen species, J. Immunol.,
181, 3850-3860.
8.Shin, J., Pan, H., and Zhong, X. P. (2012)
Regulation of mast cell survival and function by tuberous sclerosis
complex, Blood, 119, 3306-3314.
9.Zhou, Y., Tung, H. Y., Tsai, Y. M., Hsu, S. C.,
Chang, H. W., Kawasaki, H., Tseng, H. C., Plunkett, B., Gao, P., Hung,
C. H., Vonakis, B. M., and Huang, S. K. (2013) Aryl hydrocarbon
receptor controls murine mast cell homeostasis, Blood,
121, 3195-3204.
10.Chelombitko, M. A., Fedorov, A. V., Ilyinskaya,
O. P., Zinovkin, R. A., and Chernyak, B. V. (2016) The role of reactive
oxygen in mast cell degranulation, Biochemistry (Moscow),
81, 1564-1577.
11.Inoue, T., Suzuki, Y., Yoshimaru, T., and Ra, C.
(2008) Reactive oxygen species produced up- or downstream of calcium
influx regulate proinflammatory mediator release from mast cells: role
of NADPH oxidase and mitochondria, Biochim. Biophys. Acta,
1783, 789-802.
12.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L.
E., Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Y., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Y., Yaguzhinsky, L. S., Zamyatnin, A. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 1. Cationic plastoquinone derivatives:
synthesis and in vitro studies, Biochemistry (Moscow),
73, 1273-1287.
13.Popova, E. N., Pletjushkina, O. Y., Dugina, V.
B., Domnina, L. V., Ivanova, O. Y., Izyumov, D. S., Skulachev, V. P.,
and Chernyak, B. V. (2010) Scavenging of reactive oxygen species in
mitochondria induces myofibroblast differentiation, Antioxid. Redox
Signal., 13, 1297-1307.
14.Demianenko, I. A., Vasilieva, T. V., Domnina, L.
V., Dugina, V. B., Egorov, M. V., Ivanova, O. Y., Ilinskaya, O. P.,
Pletjushkina, O. Y., Popova, E. N., Sakharov, I. Y., Fedorov, A. V.,
and Chernyak, B. V. (2010) Novel mitochondria-targeted antioxidants,
“Skulachev-ion” derivatives, accelerate dermal wound
healing in animals, Biochemistry (Moscow), 75,
274-280.
15.Plotnikov, E. Y., Morosanova, M. A., Pevzner, I.
B., Zorova, L. D., Manskikh, V. N., Pulkova, N. V., Galkina, S. I.,
Skulachev, V. P., and Zorov, D. B. (2013) Protective effect of
mitochondria-targeted antioxidants in an acute bacterial infection,
Proc. Natl. Acad. Sci. USA, 110, 3100-3108.
16.Demyanenko, I. A., Popova, E. N., Zakharova, V.
V., Ilyinskaya, O. P., Vasilieva, T. V., Romashchenko, V. P., Fedorov,
A. V., Manskikh, V. N., Skulachev, M. V., Zinovkin, R. A.,
Pletjushkina, O. Yu., Skulachev, V. P., and Chernyak, B. V. (2015)
Mitochondria‐targeted antioxidant SkQ1 improves impaired dermal
wound healing in old mice, Aging (Albany, NY), 7,
475-485.
17.Shipounova, I. N., Svinareva, D. A., Petrova, T.
V., Lyamzaev, K. G., Chernyak, B. V., Drize, N. I., and Skulachev, V.
P. (2010) Reactive oxygen species produced in mitochondria are involved
in age-dependent changes of hematopoietic and mesenchymal progenitor
cells in mice. A study with the novel mitochondria-targeted antioxidant
SkQ1, Mech. Ageing Dev., 131, 415-421.
18.Manskikh, V. N., Gancharova, O. S., Nikiforova,
A. I., Krasilshchikova, M. S., Shabalina, I. G., Egorov, M. V., Karger,
E. M., Milanovsky, G. E., Galkin, I. I., Skulachev, V. P., and
Zinovkin, R. A. (2015) Age-associated murine cardiac lesions are
attenuated by the mitochondria-targeted antioxidant SkQ1, Histol.
Histopathol., 30, 353-360.
19.Demyanenko, I. A., Zakharova, V. V., Ilyinskaya,
O. P., Vasilieva, T. V., Fedorov, A. V., Manskikh, V. N., Zinovkin, R.
A., Pletjushkina, O. Y., Chernyak, B. V., Skulachev, V. P., and Popova,
E. N. (2017) Mitochondria-targeted antioxidant SkQ1 improves dermal
wound healing in genetically diabetic mice, Oxid. Med. Cell.
Longev., 2017, 1-10.
20.Zinovkin, R. A., Romaschenko, V. P., Galkin, I.
I., Zakharova, V. V., Pletjushkina, O. Y., Chernyak, B. V., and Popova,
E. N. (2014) Role of mitochondrial reactive oxygen species in
age-related inflammatory activation of endothelium, Aging (Albany,
NY), 6, 661-674.
21.Chelombitko, M. A., Averina, O. A., Vasilieva, T.
V., Dvorianinova, E. E., Egorov, M. V., Pletjushkina, O. Yu., Popova,
E. N., Fedorov, A. V., Romashchenko, V. P., and Ilyinskaya, O. P.
(2017) Comparative effects of mitochondria-targeted antioxidant
10-(6´-plastoquinonyl) decyltriphenylphosphonium bromide and a
fragment of its molecule dodecyltriphenylphosphonium on the
carrageenan-induced acute inflammation using an air pouch model in
mice, Bull. Exp. Biol. Med., 162, 730-733.
22.Zakharova, V. V., Pletjushkina, O. Yu., Galkin,
I. I., Zinovkin, R. A., Chernyak, B. V., Krysko, D. V., Bachert, C.,
Krysko, O., Skulachev, V. P., and Popova, E. N. (2017) Low
concentration of uncouplers of oxidative phosphorylation decreases the
TNF induced endothelial permeability and lethality in mice, Biochim.
Biophys. Acta, 1863, 968-977.
23.Zakharova, V. V., Pletjushkina, O. Y., Zinovkin,
R. A., Popova, E. N., and Chernyak, B. V. (2017) Mitochondria-targeted
antioxidants and uncouplers of oxidative phosphorylation in treatment
of the systemic inflammatory response syndrome (SIRS), J. Cell.
Physiol., 232, 904-912.
24.Silachev, D. N., Plotnikov, E. Y., Zorova, L. D.,
Pevzner, I. B., Sumbatyan, N. V., Korshunova, G. A., Gulyaev, M. V.,
Pirogov, Y. A., Skulachev, V. P., and Zorov, D. B. (2015)
Neuroprotective effects of mitochondria-targeted plastoquinone and
thymoquinone in a rat model of brain ischemia/reperfusion injury,
Molecules, 20, 14487-14503.
25.Jankauskas, S. S., Andrianova, N. V., Alieva, I.
B., Prusov, A. N., Matsievsky, D. D., Zorova, L. D., Pevzner, I. B.,
Savchenko, E. S., Pirogov, Y. A., Silachev, D. N., Plotnikov, E. Y.,
and Zorov, D. B. (2016) Dysfunction of kidney endothelium after
ischemia/reperfusion and its prevention by mitochondria-targeted
antioxidant, Biochemistry (Moscow), 82, 1538-1548.
26.Galkin, I. I., Pletjushkina, O. Yu., Zinovkin, R.
A., Zakharova, V. V., Chernyak, B. V., and Popova, E. N. (2016)
Mitochondria-targeted antioxidant SkQR1 reduces TNF-induced endothelial
permeability in vitro, Biochemistry (Moscow), 81,
1188-1197.
27.Galkin, I. I., Pletjushkina, O. Yu., Zinovkin, R.
A., Zakharova, V. V., Birjukov, I. S., Chernyak, B. V., and Popova, E.
N. (2014) Mitochondria-targeted antioxidants prevent TNFα-induced
endothelial cell damage, Biochemistry (Moscow), 79,
124-130.
28.Vorobjeva, N., Prikhodko, A., Galkin, I.,
Pletjushkina, O., Zinovkin, R., Sud’ina, G., Chernyak, B., and
Pinegin, B. (2017) Mitochondrial reactive oxygen species are involved
in chemoattractant-induced oxidative burst and degranulation of human
neutrophils in vitro, Eur. J. Cell. Biol., 96,
254-265.
29.Garcia-Ramallo, E., Marques, T., Prats, N.,
Beleta, J., Kunkel, S. L., and Godessar, N. (2002) Resident cell
chemokine expression serves as the major mechanism for leukocyte
recruitment during local inflammation, J. Immunol., 169,
6467-6473.
30.Sin, Y. M., Sedgwick, A. D., Chea, E. P., and
Willoughby, D. A. (1986) Mast cells in newly formed lining tissue
during acute inflammation: a six day air pouch model in the mouse,
Ann. Rheum. Dis., 45, 873-877.
31.Romano, M., Faggioni, R., Sironi, M., Sacco, S.,
Echtenacher, B., Di Santo, E., Salmona, M., and Ghezzi, P. (1997)
Carrageenan-induced acute inflammation in the mouse air pouch synovial
model. Role of tumour necrosis factor, Mediators Inflamm.,
6, 32-38.
32.Duarte, D. B., Vasko, M. R., and Fehrenbacher, J.
C. (2016) Models of inflammation: carrageenan air pouch, Curr.
Protoc. Pharmacol., 72, 1-9.
33.Shore, P. A., Burkhalter, A., and Cohn, V. H.
(1959) A method for the fluorometric assay of histamine in tissues,
J. Pharmacol. Exp. Ther., 127, 182-186.
34.Barsumian, E. L., Isersky, C., Petrino, M. G.,
and Siraganian, R. P. (1981) IgE-induced histamine release from rat
basophilic leukemia cell lines: isolation of releasing and nonreleasing
clones, Eur. J. Immunol., 11, 317-323.
35.McShane, M. P., Friedrichson, T., Giner, A.,
Meyenhofer, F., Barsacchi, R., Bickle, M., and Zerial, M. (2015) A
combination of screening and computational approaches for the
identification of novel compounds that decrease mast cell
degranulation, J. Biomol. Screen., 20, 720-728.
36.Radinger, M., Jensen, B. M., Swindle, E., and
Gilfillan, A. M. (2015) Assay of mast cell mediators, Methods Mol.
Biol., 1220, 307-323.
37.Oliani, S. M., Lim, L. H. K., Christian, H. C.,
Pell, K., Das, A. M., and Perretti, M. (2001) Morphological alteration
of peritoneal mast cells and macrophages in the mouse peritoneal cavity
during the early phases of an allergic inflammatory reaction, Cell.
Biol. Int., 25, 795-803.
38.Benly, P. (2015) Role of histamine in acute
inflammation, J. Pharm. Sci. Res., 7, 373-376.
39.Hartveit, F., and Thunold, S. (1966) Peritoneal
fluid volume and the estrus cycle in mice, Nature, 210,
1123-1125.
40.Cassado, A. A., D’Imperio, L. M. R., and
Bortoluci, K. R. (2015) Revisiting mouse peritoneal macrophages:
heterogeneity, development, and function, Front. Immunol.,
6, 1-9.
41.Rashid, A., Sadroddiny, E., Ye, H. T., Vratimos,
A., Sabban, S., Carey, E., and Helm, B. (2012) Review: diagnostic and
therapeutic applications of rat basophilic leukemia cells, Mol.
Immunol., 52, 224-228.
42.Kolaczkowska, E., Seljelid, R., and Plytycz, B.
(2001) Role of mast cells in zymosan-induced peritoneal inflammation in
Balb/c and mast cell-deficient WBB6F1 mice, J. Leukoc. Biol.,
69, 33-42.
43.Ajuebor, M. N., Das, A. M., Virag, L., Flower, R.
J., Szabo, C., and Perretti, M. (1999) Role of resident peritoneal
macrophages and mast cells in chemokine production and neutrophil
migration in acute inflammation: evidence for an inhibitory loop
involving endogenous IL-10, J. Immunol., 162,
1685-1691.
44.Dahdah, A., Gautier, G., Attout, T., Fiore, F.,
Lebourdais, E., Msallam, R., Daeron, M., Monteiro, R. C., Benhamou, M.,
Charles, N., Davoust, J., Blank, U., Malissen, B., and Launay, P.
(2014) Mast cells aggravate sepsis by inhibiting peritoneal macrophage
phagocytosis, J. Clin. Invest., 24, 4577-4589.
45.Norozian, F., Kashyap, M., Ramirez, C. D., Patel,
N., Kepley, C. L., Barnstein, B. O., and Ryan, J. J. (2006) TGFbeta1
induces mast cell apoptosis, Exp. Hematol., 34,
579-587.
46.Melendez, G. C., Voloshenyuk, T. G., McLarty, G.
L., Levick, S. P., and Brower, G. L. (2010) Oxidative stress-mediated
cardiac mast cell degranulation, Toxicol. Environ. Chem.,
92, 1293-1301.
47.Jamur, M. C., Moreno, A. N., Mello, L. F. C.,
Junior, D. A. S., Campos, M. R. C., Pastor, M. V. D., Grodzki, A. C.
G., De Silva, C., and Oliver, C. (2010) Mast cell repopulation of the
peritoneal cavity: contribution of mast cell progenitors versus bone
marrow derived committed mast cell precursors, BMC Immunol.,
11, 1-12.
48.Swindle, E. J., and Metcalfe, D. D. (2007) The
role of reactive oxygen species and nitric oxide in mast cell dependent
inflammatory processes, Immunol. Rev., 217, 186-205.
49.Chen, S., Gong, J., Liu, F., and Mohammed, U.
(2000) Naturally occurring polyphenolic antioxidants modulate
IgE-mediated mast cell activation, Immunology, 100,
471-480.
50.Suzuki, Y., Yoshimaru, T., Matsui, T., Inoue, T.,
Niide, O., Nunomura, S., and Ra, C. (2003) FceRI signaling of mast
cells activates intracellular production of hydrogen peroxide: role in
the regulation of calcium signals, J. Immunol., 171,
6119-6127.
51.Matsui, T., Suzuki, Y., Yamashita, K., Yoshimaru,
T., Suzuki-Karasaki, M., Hayakawa, S., Yamaki, M., and Shimizu, K.
(2000) Diphenyleneiodonium prevents reactive oxygen species generation,
tyrosine phosphorylation, and histamine release in RBL-2H3 mast cells,
Biochem. Biophys. Res. Commun., 276, 742-748.
52.Masinia, E., Banib, D., Vannaccia, A.,
Pierpaolia, S., Mannaionia, P. F., Comhairc, S. A. A., Xuc, W.,
Muscolid, C., Erzurumc, S. C., and Salveminie, D. (2005) Reduction of
antigen induced respiratory abnormalities and airway inflammation in
sensitized guinea pigs by a superoxide dismutase mimetic, Free
Radic. Biol. Med., 39, 520-531.
53.Han, S. Y., Bae, J. Y., Park, S. H., Kim, Y. H.,
Park, J. H. Y., and Kang, Y. H. (2013) Resveratrol inhibits
IgE-mediated basophilic mast cell degranulation and passive cutaneous
anaphylaxis in mice, J. Nutr., 143, 632-639.
54.Tagen, M., Elorza, A., Kempuraj, D., Boucher, W.,
Kepley, C. L., Shirihai, O. S., and Theoharides, T. C. (2009)
Mitochondrial uncoupling protein 2 inhibits mast cell activation and
reduces histamine content, J. Immunol., 183,
6313-6319.
55.Zhang, B., Alysandratos, K. D., Angelidou, A.,
Asadi, S., Sismanopoulos, N., Delivanis, D. A., Weng, Z., Miniati, A.,
Vasiadi, M., Katsarou-Katsari, A., Miao, B., Leeman, S. E.,
Kalogeromitros, D., and Theoharides, T. C. (2011) Human mast cell
degranulation and preformed TNF secretion require mitochondrial
translocation to exocytosis sites: relevance to atopic dermatitis,
J. Allergy Clin. Immunol., 127, 1522-1531.
56.Pletjushkina, O. Y., Lyamzaev, K. G., Popova, E.
N., Nepryakhina, O. K., Ivanova, O. Y., Domnina, L. V., Chernyak, B.
V., and Skulachev, V. P. (2006) Effect of oxidative stress on dynamics
of mitochondrial reticulum, Biochim. Biophys. Acta, 1757,
518-524.
57.Wu, S., Zhou, F., Zhang, Z., and Xing, D. (2011)
Mitochondrial oxidative stress causes mitochondrial fragmentation via
differential modulation of mitochondrial fission-fusion proteins,
FEBS J., 278, 941-954.