REVIEW: Three-Finger Proteins from the Ly6/uPAR Family: Functional Diversity within One Structural Motif
N. A. Vasilyeva1,2,3, E. V. Loktyushov1,2, M. L. Bychkov2, Z. O. Shenkarev2, and E. N. Lyukmanova1,2*
1Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: ekaterina-lyukmanova@yandex.ru2Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia
3Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, 117485 Moscow, Russia
* To whom correspondence should be addressed.
Received September 7, 2017
The discovery in higher animals of proteins from the Ly6/uPAR family, which have structural homology with snake “three-finger” neurotoxins, has generated great interest in these molecules and their role in the functioning of the organism. These proteins have been found in the nervous, immune, endocrine, and reproductive systems of mammals. There are two types of the Ly6/uPAR proteins: those associated with the cell membrane by GPI-anchor and secreted ones. For some of them (Lynx1, SLURP-1, SLURP-2, Lypd6), as well as for snake α-neurotoxins, the target of action is nicotinic acetylcholine receptors, which are widely represented in the central and peripheral nervous systems, and in many other tissues, including epithelial cells and the immune system. However, the targets of most proteins from the Ly6/uPAR family and the mechanism of their action remain unknown. This review presents data on the structural and functional properties of the Ly6/uPAR proteins, which reveal a variety of functions within a single structural motif.
KEY WORDS: three-finger proteins, nicotinic acetylcholine receptor, Ly6/uPAR, Lynx1, Lypd6, SLURPDOI: 10.1134/S0006297917130090
Abbreviations: α-Bgtx, α-bungarotoxin; ACh, acetylcholine; GABA, gamma-aminobutyric acid; GPI-anchor, glycophosphatidylinositol anchor; Ly6, lymphocyte antigen 6; mAChR, muscarinic acetylcholine receptor; nAChR, nicotinic acetylcholine receptor; tPA, tissue plasminogen activator; uPAR, urokinase plasminogen activator receptor; WTX, nonconventional toxin from Naja kaouthia.
The Ly6/uPAR family got its name from the names of two representatives:
lymphocyte antigen-6 (Ly6) and urokinase-type plasminogen activator
receptor (uPAR). Proteins from this family are characterized by
presence of one or several LU domains consisting of 60-90 amino acids
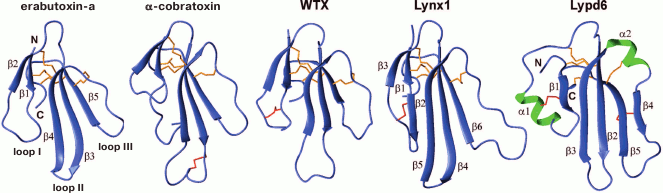
(a.a.) (Fig. 1). The LU domain includes a
β-structural core, stabilized by a system of four invariant
disulfide bonds, and three extended loops (Fig. 2). Thereby, the Ly6/uPAR proteins are often called
three-finger proteins. The functional diversity of the Ly6/uPAR
proteins is connected with variability of the loop regions. The loop
regions can also contain additional disulfide bonds (Fig. 2).
Fig. 1. Comparison of amino acid sequences of three-finger proteins of different origin. Conservative Cys residues are shown in yellow. Cys residues participating in the formation of additional disulfide bonds are shown in green. Additional N- and C-terminal sequences of Lypd6 and Lypd6b are shown in blue. A single asterisk indicates protein sequences for which presence of a GPI-anchored form is shown, and two asterisks show proteins for which presence of both GPI-anchored and secreted form is reported.
Fig. 2. Structure of the LU domains of the three-finger erabutoxin-a from Laticauda semifasciata (PDB code 1QKD), α-cobratoxin from Naja kaouthia (PDB code 2CTX), WTX[P33A] from Naja kaouthia (PDB code 2MJO), human Lynx1 (PDB code 2LO3) and human Lypd6 (Shenkarev et al., in preparation for publication). Conservative and additional disulfide bonds are shown in orange and red, respectively.
To date, the Ly6/uPAR proteins have been found in insects [1], fish [2], amphibians [3], reptiles [4], birds [5], and mammals [6]. The best-known three-finger proteins are snake neurotoxins, which act on numerous targets such as nicotinic acetylcholine receptors (nAChRs), muscarinic acetylcholine receptors (mAChRs), α/β-adrenergic receptors, gamma-aminobutyric acid (GABA) receptors, acid-sensitive ion channels (ASIC), etc. [7]. Conservation of the three-finger structural motif points on the great functional significance of the mammalian Ly6/uPAR proteins. In the human genome, there are 35 genes encoding three-finger proteins [8], most of which remain poorly studied. Ly6/uPAR proteins can be associated with the cell membrane by glycosylphosphatidylinositol anchor (GPI anchor), and they can be secreted. In this review, the Ly6/uPAR proteins of insects and chordates are considered, and information about the function of mammalian three-finger proteins is discussed.
SNAKE TOXINS
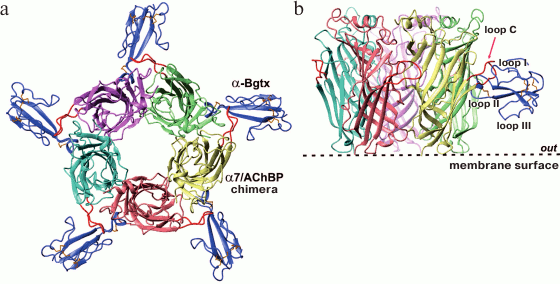
α-Neurotoxins and nicotinic acetylcholine receptors. Toxins are major protein components of snake venoms. α-Neurotoxins, the best-studied snake toxins, are highly specific inhibitors of nAChRs, which are ligand-gated ion channels. α-Neurotoxins are considered as tools for studying nAChRs properties, and as prototypes of drugs for the treatment of diseases of the nervous system [9]. Snake α-neurotoxins contain 60-75 a.a. and 4-5 disulfide bonds. There are short-chain α-neurotoxins (60-62 a.a., 4 disulfide bonds) and long-chain α-neurotoxins (66-75 a.a., having a fifth disulfide bond in the central loop; Fig. 2). Both types of α-neurotoxins efficiently interact with muscle type nAChRs, but only the long-chain α-neurotoxins act on neuronal α7-nAChRs [10]. It was revealed that the main structural motif of the α-neurotoxins interacting with nAChRs is the tip of the central loop. The fifth disulfide bond in the central loop is essential for the interaction with α7-nAChRs (Fig. 3) [10, 11].
Fig. 3. Structure of the α-bungarotoxin (α-Bgtx) complex with a chimeric protein homologous to the extracellular domain of α7-nAChR (PDB 4HQP) [11]. The top view (a) and the side view (b) are shown. Different subunits of the receptor are shown in different colors. Red color shows the C-loop of the receptor closing the entrance to the orthosteric ligand-binding site.
Neurotoxins and muscarinic acetylcholine receptors. Neurotoxins isolated from snake venom can also target mAChRs related to G-protein coupled receptors (GPCR) [12]. There are muscarinic neurotoxins isolated from mamba venom (MT1-MT7, four disulfide bonds) that interact with different types of mAChRs as agonists, antagonists, and allosteric modulators [12]. Allosteric antagonists MT3 and MT7 isolated from Dendroaspis angusticeps demonstrate high specificity for M1 and M4 mAChRs, whereas other muscarinic toxins are less specific [12]. It was shown that the central loop of MT7 is the main structural determinant of high-affinity interaction with M1 mAChRs [13].
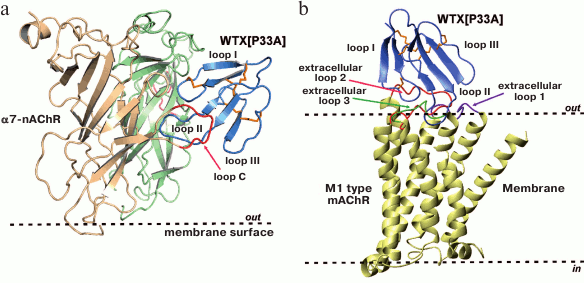
Non-conventional toxins. There are about 30 non-conventional toxins isolated from Elapidae venoms having an additional fifth disulfide in the loop I. The function and targets of these toxins have been poorly studied [14]. In general, these non-conventional toxins are characterized by lower toxicity (LD50 = 5-80 mg/kg) than α-neurotoxins (LD50 = 0.04-0.30 mg/kg) [14]. However, there are also highly toxic non-conventional toxins, for example, γ-bungarotoxin isolated from B. multicinctus (LD50 ~ 0.15 mg/kg). Phylogenetic analysis revealed that the family of non-conventional toxins is not homogeneous. Thus, some (mainly those from Naja spp.) have a close relationship with muscarinic mamba toxins, while toxins isolated from Bungarus spp. have a close relationship with long-chain α-neurotoxins [4]. Non-conventional (weak) toxin WTX isolated from Naja kaouthia (LD50 > 2 mg/kg) combines the properties of α-neurotoxins and muscarinic toxins. WTX irreversibly blocks with low affinity (IC50 ~ 10 µM) muscle type and α7-nAChRs, but can also interact with different types of mAChRs as allosteric modulator [15]. The central loop of WTX, which demonstrates high conformational plasticity, is important for interaction with both nAChRs and mAChRs (Fig. 4, [16, 17]).
Fig. 4. Models of complexes of non-conventional toxin WTX with the extracellular domain of α7-nAChR (a) and M1 type mAChR (b). In the case of nAChR, the central loop of the toxin interacts with the orthosteric binding site of the receptor [16], and in the case of mAChR it interacts with the allosteric binding site [17].
Neurotoxins and GABAA-receptors. Recently, the three-finger toxins MmTX1 and MmTX2 increasing the sensitivity of γ-aminobutyric acid receptors (GABAA receptors) to agonists were isolated from Micrurus mipartitus snake venom [18]. α-Bgtx, previously known as a highly specific nAChR inhibitor, was found to selectively inhibit GABAA-evoked currents through the channel of the α2β2γ2 receptor, although with significantly less efficacy [19]. The ability of three-finger neurotoxins to inhibit GABAA receptors has also been demonstrated in the case of α-cobratoxin, neurotoxin I, and toxin WTX [20]. It turned out that an important structural determinant of neurotoxin I for interaction with GABAA receptors is the central loop, as in the case with nAChRs and mAChRs [20].
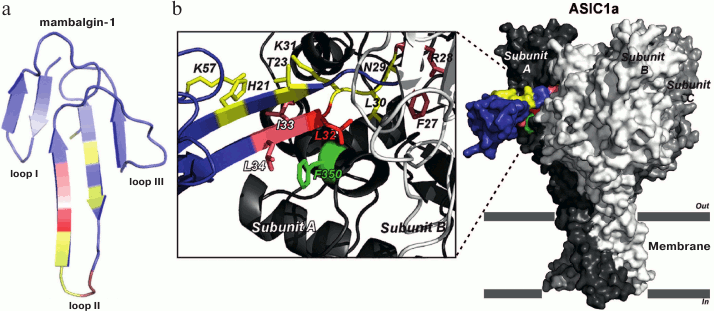
Neurotoxins and acid-sensitive ion channels (ASIC). Two three-finger proteins called mambalgin-1 and mambalgin-2 demonstrating analgesic effect comparable with the effect of morphine were isolated from black mamba venom [21]. Mambalgins target the acid-sensitive channels (ASIC) responsible for pain in mammals (ASIC1a, ASIC2a, and ASIC1b) [21]. Recently, the crystal structure of mambalgin-1 was determined. It has been shown that the binding site of the peptide to the ASIC1a channel is located in the central loop (amino acid residues Phe27, Leu32, and Leu34; Fig. 5) [22].
Fig. 5. Model of the complex of mambalgin-1 with the ASIC1a channel. The crystal structure of mambalgin-1 (a) and contacts of the toxin central loop with the channel (b) are shown. The figure was adapted from [22].
Cytotoxins. The target of three-finger cytotoxins is the cell membrane. The tips of the cytotoxin loops are rich in hydrophobic amino acid residues forming a membrane-binding motif [23]. The cytotoxins interact with anionic lipids of the membrane of various cells, for example of myocytes, and they induce the disintegration of the cell membrane [24]. An antitumor activity of cytotoxins associated with their accumulation in lysosomes was shown on lung carcinoma A549 and leukemia HL60 cells. When the concentration of cytotoxins in lysosomes exceeds some threshold value, the lysosomal membrane is disrupted, thus initiating apoptosis or necrosis of cancer cells [25].
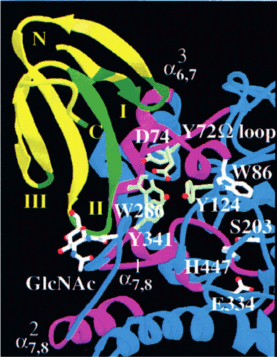
Inhibitors of acetylcholinesterase. Fasciculins from mamba venom prevent neuromuscular transmission by inhibiting acetylcholinesterase, which hydrolyzes ACh in the synaptic cleft [26]. The fasciculin structure is similar to that of short α-neurotoxins (Fig. 2). Fasciculins interact with acetylcholinesterase by the first and second toxin loops (Fig. 6). The first loop provides a larger interaction area with the enzyme and the second loop rich in hydrophobic residues blocks the access of the substrate (ACh) to the acetylcholinesterase catalytic site [27].
Fig. 6. Structure of the complex of fasciculin-2 with acetylcholinesterase. The second loop of fasciculin-2 interacts with the peripheral anionic site of the enzyme, whereas the loop I binds to the acetylcholinesterase Ω-loop. The sites of fasciculin and the enzyme involved in the complex formation are shown in green and purple colors, respectively. The figure was adapted from [27].
INSECT NEUROMODULATORS
Sleepless, a regulator of sleep in Drosophila. The sleepless gene has been identified in Drosophila. It encodes a three-finger protein responsible for sleep. The SLEEPLESS protein is anchored to cell membrane of neurons by a GPI anchor and is highly expressed in the brain [1]. The loss of SLEEPLESS causes a significant decrease in sleep duration. A moderate decrease in SLEEPLESS expression weakly affects the baseline sleep, but significantly reduces the recovery sleep following sleep deprivation. The known mutation quiver, which affects the current through the K+ channel Shaker, is located at the sleepless gene. The expression of Shaker in sleepless mutants is decreased [1], while SLEEPLESS enhances Shaker expression and directly interacts with the ion channel, increasing the open probability [1, 28]. In addition, SLEEPLESS is an antagonist of nAChRs [28]. Both factors lead to reduced excitability of nerve cells as well as to a decrease in synaptic transmission, which in turn results in a transition from wakefulness to sleep [28]. The key role of the SLEEPLESS central loop in the interaction with both nAChRs and Shaker has been revealed [29].
Lynx1 and lynx2 from the brown planthopper Nilaparvata lugens. The nAChRs, target for neonicotinoid insecticides, mediate rapid cholinergic synaptic transmission in the insect brains. Two three-finger proteins Nl-lynx1 and Nl-lynx2 from the brown planthopper Nilaparvata lugens modulate the nAChR activity, increasing agonist-induced macroscopic currents through the Nlα1/β2 heteromeric receptors, but do not change receptor sensitivity to agonists and desensitization properties [30]. The Y151S mutation of the α1 nAChR subunit increases insect resistance to neonicotinoid insecticides (imidacloprid). It has been shown that Nl-lynx1 and Nl-lynx2 proteins significantly increase the amplitude of imidacloprid-evoked current at mutant Nlα1/β2 receptors, but do not affect the ACh-evoked current [30]. Thus, Nl-lynx1 and Nl-lynx2 can act as factors affecting the sensitivity of insects to neonicotinoid insecticides [30].
THREE-FINGER PROTEINS FROM FISH AND AMPHIBIANS
Li16 protein of the tree frog Rana sylvatica. The tree frog Rana sylvatica experiences a many-week winter hibernation with freezing of the body up to 65%. Freezing involves adaptation mechanisms that prevent long-term ischemia and protect macromolecules from disruption. The gene li16 encoding 89-a.a. protein with five disulfide bonds was found in the tree frog (Fig. 1, [31]). The level of Li16 expression significantly increases during the first day of freezing. Hypoxia and dehydration also lead to increase of Li16 expression. Defrosting and restoration of the normal oxygen level lead to decline in li16 gene expression to the control level, that possibly points to the important role of Li16 in the development of resistance to ischemia during freezing [31].
Prod1 protein, a regulator of limb regeneration in salamandra. Prod1 is a membrane-tethered three-finger protein that regulates regeneration in salamanders, determining the direction of limb growth [3]. Impaired prod1 expression blocks the formation of the radius and ulna and outgrowth of the anterior digits [32]. Recently, it was shown that Prod1 plays an important role in the regulation of cell adhesion. Membrane-bound Prod1 molecules aggregate in the cell membrane and interact with Prod1 molecules on the membrane of neighboring cells, triggering cell adhesion during limb regeneration [33].
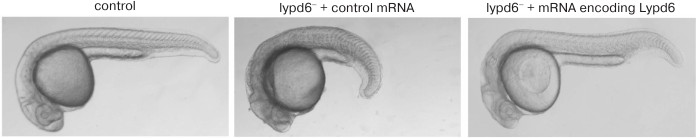
Lypd6 protein, a regulator of fish and frog embryogenesis. During the development of zebrafish Danio rerio, lypd6 expression was found at the stages of blastula, gastrula, segmentation, and organogenesis [2]. It was shown that the three-finger protein Lypd6 enhances the Wnt/β-catenin signaling, which regulates embryogenesis and cell differentiation [2]. The overexpression of the Wnt/β-catenin signaling inhibitors Axin1 and Dkk1 suppresses the lypd6 expression at the gastrula and somitogenesis embryonal stages, and the overexpression of the signal activator Wnt8 in contrast increases the lypd6 expression at the somitogenesis stage. Blocking of lypd6 expression using morpholine oligonucleotides leads to morphological changes in embryos of Danio rerio, but the co-injection of Lypd6 mRNA restores normal development of the embryos (Fig. 7). Lypd6 tethering in membrane rafts by GPI anchor was shown to be necessary for interaction with the Lrp6/Frizzled8 receptor complex and activation of Lrp6 phosphorylation in the membrane rafts [2]. A Lypd6 protein homolog was also found in Xenopus frogs (Xlypd6; Fig. 1). Expression profile of Xlypd6 during embryogenesis resembles the expression profile of Lypd6 in Danio rerio [2].
Fig. 7. Blocking of lypd6 expression using morpholine oligonucleotides (lypd6–) leads to morphological changes in embryos of Danio rerio. Co-injection of mRNA encoding lypd6 restores the morphology of the embryo. The figure was adapted from [2].
Recently, a cluster of genes encoding three secreted three-finger proteins was found in chromosome 2 of Danio rerio. The function of these proteins is currently unknown, but expression in the endoderm indicates their involvement in the development of internal organs [34].
THREE-FINGER PROTEINS OF MAMMALS
Glycoprotein CD59 – an inhibitor of membrane attack complex or protectin. CD59 is a membrane-bound regulatory protein of the complement system. This protein is found in various blood, epithelial, endothelial, and placenta cells. Soluble forms of CD59 have been found in saliva, amniotic fluid, and urine [35]. Mutations of the cd59 gene or genes of enzymes responsible for the synthesis of glycosylphosphatidylinositol lead to partial or complete absence of CD59 protein. As a result, red blood cells undergo complement-mediated lysis, which can lead to the development of hemolytic anemia [36]. The spatial structure of human CD59, determined by NMR spectroscopy in 1994, was the first proof that three-finger proteins are expressed in mammals [35]. The protein has a three-finger structure characteristic for snake neurotoxins, but unlike β-structural toxins, the elongated CD59 third loop contains an α-helical region.
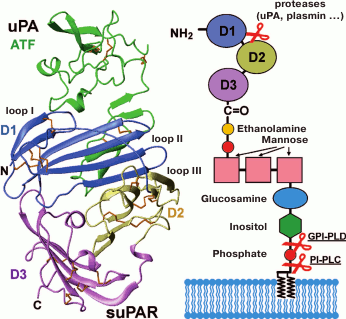
Urokinase-type plasminogen activator receptor (uPAR). The urokinase uPA receptor is synthesized by normal and tumor cells and presents on monocytes, fibroblasts, platelets, and endothelium. The uPA/uPAR system plays an important role in cell proliferation, differentiation, adhesion, migration, plasminogen activation, and remodeling of extracellular matrix and basal membrane [37]. uPAR is bound to the membrane by GPI anchor, but there is also a soluble form of the receptor – suPAR (Fig. 8). A high level of suPAR is a negative prognosis of tumor disease, and uPAR inhibition may be a strategy for cancer treatment [38].
Fig. 8. Structure of the complex of the N-terminal fragment of uPA (ATF) and water-soluble uPAR (suPAR, PDB 2FD6) [39]. The three LU domains of the uPAR molecule (D1, D2, D3) are shown in different colors. GPI anchor linked to the C-terminal sequence of uPAR attaches the receptor to the cell membrane. On the right, a schematic structure of uPAR containing the GPI anchor is shown. The sites of possible uPAR molecule cleavage that could lead to the formation of active soluble signal forms of the receptor are shown.
The uPA receptor consists of three LU domains – D1, D2, and D3 (Fig. 8) [39]. These domains participate in the interaction with the N-terminal domain of uPA (ATF), which activates plasminogen. Domains D2 and D3 can be cleaved out and interact with the lipoxin A4 receptor (LXA4R) transmitting chemotaxis signals [37]. In addition, uPAR can bind to integrins that promotes intercellular interactions and adhesion [37].
Lynx1 is a factor of neuronal plasticity in mammals. Lynx1 was the first three-finger protein found in the CNS of mammals [6]. Lynx1 is expressed in the Purkinje cells, cerebellum nuclei, cerebral cortex, and hippocampus [6]. Moreover, co-localization of Lynx1 attached to the neuronal membrane by GPI anchor and nAChRs was detected in the cerebral cortex, thalamus, substantia nigra, cerebellum, hippocampus, and amygdala [40]. In addition, Lynx1 is expressed by GABA-ergic parvalbumin interneurons of the visual cortex in mouse brain [41].
Co-expression of membrane-anchored Lynx1 with α4β2-nAChRs in mammalian cell culture leads to an increase in the time and degree of receptor desensitization, as well as channel conductivity [40]. In the endoplasmic reticulum, Lynx1 affects the assembly of α4β2-nAChRs by stabilizing α4/α4, but not β2/β2 dimers. This results in a shift of the receptor stoichiometry to (α4)3(β2)2-nAChRs. They have reduced sensitivity to ACh compared with (α4)2(β2)3-nAChRs [42].
The neurons of lynx1 knockout mice are more sensitive to nicotine. Knockout mice receiving nicotine demonstrated an increased sensitivity in the fear conditioning test and an enhanced motor activity in the rotarod test [43]. Transgenic mice with overexpression of the secreted form of Lynx1 (without the GPI anchor) also showed increased motor learning ability, whereas mice with overexpression of GPI-anchored Lynx1 did not. Thus, the soluble form of Lynx1 can act as a potential tool for modulating of ACh-dependent brain plasticity and learning mechanisms [44].
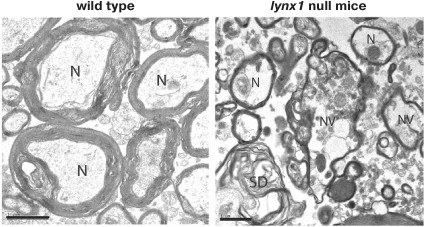
Knockout mice have displayed the destruction of nerve bundles, the loss of nerve fibers and neuronal nuclei, as well as vacuolization and destruction of myelin sheath during aging in the dorsal striatum (Fig. 9). In contrast, these morphological features were not observed in young transgenic mice and in heterozygous mice of any age. This suggests the possibility of maintaining neuronal health with increasing concentration of Lynx1 [45].
Fig. 9. Breakdown in nerve fiber (N) bundles in 13-month-old lynx1 null mice. In lynx1 null mice an increase in the distance between the fibers, disordered myelin sheaths (denoted as SD), and intracellular vacuolization of the axon (designated as NV) are observed. The bar scale is 1 µm. The figure was adapted from [45].
It is known if a juvenile animal has one eye closed for several weeks (monocular deprivation) and then it is opened after the end of a critical period, the vision in this eye is not restored. In the case of adult lynx1 knockout mice, complete restoration of vision was observed after the eye was opened [46]. Monocular deprivation leads to increased activity of tissue plasminogen activator (tPA) in the primary visual cortex. Normally, tPA activity does not change in adult animals [47]. Adult lynx1 knockout mice demonstrated increased activity of tPA accompanied by a decrease in dendrite spine density and a change in the formation of ocular dominance [47]. Deletion of lynx1 was shown to increase the rate of appearance and disappearance of dendrite spines in the visual cortex of adult animal brains [48]. Thus, Lynx1 is one of the key factors regulating neuronal plasticity.
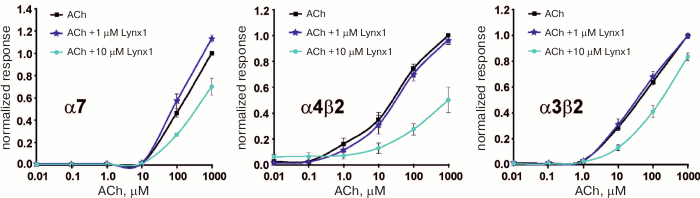
Soluble analog of Lynx1. The development of the bacterial expression system for the water-soluble domain of Lynx1 without a GPI anchor [49] made possible detailed study of its structural and functional properties. For example, previously unknown targets of Lynx1 were found (α3β2-nAChRs, muscular nAChRs and M3 mAChRs). It was shown that Lynx1 at low concentration (1 µM) activates and at high concentration (10 µM) inhibits α7-nAChRs, while only inhibitory effect of Lynx1 was demonstrated at α3β2 and α4β2 nAChRs (Fig. 10). It was found that Lynx1 interacts with nAChRs in an allosteric mode [50].
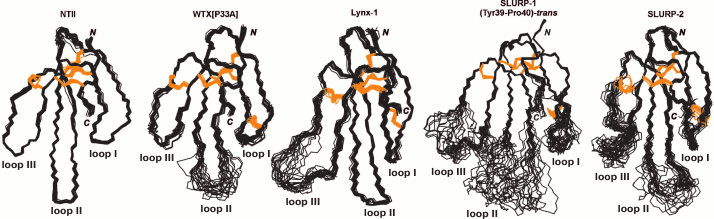
Using NMR spectroscopy, it was shown that Lynx1, like α-neurotoxins, has a three-finger β-structural spatial organization [50]. However, in contrast to snake neurotoxins, the extended third loop of Lynx1, possibly interacting with the receptor, demonstrates great conformational mobility in the ps-ns time range and does not have an ordered structure in solution (Fig. 11). Similar dynamic properties have been described for the central loop of the non-conventional toxin WTX, which can interact with low affinity with both nAChRs and mAChRs [17]. Perhaps, the high plasticity of the loops is one of the factors that determines simultaneously the ability to interact with different molecular targets and to have a low affinity for them. For comparison, snake α-neurotoxins with more ordered loops (Fig. 11) inhibit nAChRs with significantly greater affinity [9]. Based on the Lynx1 structure, models of the complexes of the neuromodulator with nAChR have been proposed. According to the models of these complexes, Lynx1 interacts with the outside of the receptor loop C without penetration into the orthosteric binding site and does not prevent the interaction between the receptor and agonists [50, 51].
Fig. 10. Effect of the water-soluble LU-domain of Lynx1 on ACh-induced currents on α3β2, α4β2, and α7-nAChR expressed in Xenopus oocytes. The figure was adapted from [50].
Fig. 11. Superposition of spatial structures determined by NMR spectroscopy for neurotoxin II from Naja oxiana (NTII), WTX[P33A], Lynx1, SLURP-1, and SLURP-2 (codes in the PDB database 1NOR, 2MJO, 2LO3, 2MUO, and 2N99, respectively). Disulfide bonds are shown in orange color.
Role of Lynx1 in pathophysiology of Alzheimer’s disease. One of the reasons for cognitive impairment in the brain of patients with Alzheimer’s disease is nAChRs dysfunction [52]. The co-expression of Lynx1 and nAChRs in the brain regions responsible for memory and learning [53] suggests the involvement of Lynx1 in the development of Alzheimer’s disease [54]. It was shown that water-soluble Lynx1 competes with oligomeric β-amyloid peptide (1-42) for binding to nAChR subunits isolated from the human brain homogenate. Moreover, the preincubation of cultured cortical neurons with the water-soluble Lynx1 significantly reduced the cytotoxic effect of β-amyloid peptide (1-42) [54]. On the other hand, it was found that the expression level of Lynx1 in the cerebral cortex of transgenic mice with β-amyloid and tau pathology is significantly lower compared with healthy mice [54]. Perhaps, the cognitive function impairment in Alzheimer’s disease is associated with a decreased Lynx1 expression level.
Lynx1 modulates cholinergic activity in healthy and tumor cells of bronchial epithelium. Besides the brain, Lynx1 expression was also detected, although in much lower amounts, in lung tissue [53]. Lynx1 has been shown to act as a negative modulator of nAChRs in healthy and tumor lung tissues [55]. In healthy lung, Lynx1 downregulates the increased expression of nAChRs and GABA receptors observed upon chronic nicotine treatment, and it controls mucin synthesis [55]. A decreased Lynx1 expression is observed in lung cancer. Blocking of lynx1 gene expression by interfering RNA stimulates cancer cell growth, while lynx1 overexpression reduces cell proliferation [55]. Thus, Lynx1 can be considered as a prototype for drugs for asthma, chronic obstructive pulmonary disease, and lung cancer treatment [55]. Interestingly, in addition to Lynx1, expression of the neuromodulator Lynx2 was also observed in lung tissues [55].
Lynx2 neuromodulator. The expression of lynx2 was first detected in neurons of the peripheral and central nervous systems in mouse embryos [56]. A high level of lynx2 expression was observed in the hippocampal CA1 region, dentate gyrus neurons, deep layers of cerebral cortex, and spinal cord neurons [56]. The lynx2 mRNA was found in the visual cortex [46], hippocampus, and frontal cortex [57]. During the first two weeks of the postnatal period, the lynx2 mRNA level in the frontal cortex and hippocampus increases, followed by a gradual decline that emerges on a plateau at 60- and 26-day age, respectively [53].
It was shown that Lynx2 reduces ACh-induced current through α4β2-nAChRs expressed in Xenopus oocytes and enhances their desensitization [57]. On the other hand, Lynx2 reduces α4β2-nAChRs expression on the cell membrane by preferentially forming a complex with α4, rather than the β2 receptor subunit [58]. Perhaps, Lynx2 affects the α4β2-nAChR stoichiometry during the receptor assembly in the endoplasmic reticulum, as shown previously for Lynx1 [42].
Despite having similar pharmacological properties, lynx1 and lynx2 expression profiles in the brain differ significantly. This probably indicates the involvement of these neuromodulators in different processes. This hypothesis is supported by experiments with lynx2 knockout mice demonstrating normal motor and sensorimotor activity compared to the wild type [57] in contrast to lynx1 knockout mice [43].
Neuromodulator lypd6. The expression of the lypd6 gene encoding a membrane-tethered three-finger protein was found in the cerebral cortex and the spinal cord in mice [59]. In rats, lypd6 expression was found in the brain, lung, kidneys, heart, liver, and prostate [60]. In humans, lypd6 expression was demonstrated in different tissues, especially in the brain and heart [61]. Expression of lypd6, as well as lynx1, was shown in the GABA-ergic interneurons of the mouse visual cortex, although the lynx1 expression was observed only in the parvalbumin interneurons, while lypd6 expression was revealed only in the somatostatin interneurons [41]. In the serotonin-expressed interneurons, neither lynx1, nor lypd6 mRNA was detected [41]. Differentiation of the expression patterns of various neuromodulators in the brain regions can be of great importance for directed and specific modulation of individual populations of interneurons in the treatment of mental disorders [41].
It has been shown that Lypd6 increases the amplitude of the Ca2+ current in response to nicotine in the neurons of the mice trigeminal ganglion [59]. In line with this, transgenic mice with Lypd6 overexpression demonstrate increased locomotor activity and visceral hyperalgesia, indicating an increase in cholinergic tone [59]. Using inhibitors of α7-nAChR, it was found that the target of Lypd6 is not homopentameric α7-nAChRs, but other types of nAChRs [59]. In contrast, the water-soluble recombinant analog of Lypd6 fused with glutathione-S-transferase inhibits the nicotine-induced current in the CA1 region in the hippocampal slices [60]. The discrepancy in the functional activity of endogenous and recombinant neuromodulators may be due to presence of glutathione-S-transferase in the recombinant protein. A similar effect of additional sequences on neuromodulator activity against nAChRs has also been observed for human SLURP-1 [62].
Nicotine administration to rats in the prenatal and early postnatal period resulted in an increased Lypd6 expression level in the hippocampus, which was not observed in adult animals [60]. At the same time, significant changes in the Lynx1 and nAChR β2 subunit expression profiles were not detected [60]. Attempts to obtain mice with blocked lypd6 expression led either to death or to infertile animals [59]. This, taken together with the high homology of the amino acid sequences of mouse and Danio rerio Lypd6 (~87%; Fig. 1), possibly indicates the special role of Lypd6 and associated with it cholinergic activation in embryonic development.
Neuromodulator lypd6b. The lypd6b gene expression was found in testes, lungs, stomach, prostate, brain, and other human organs [63]. Lypd6b is expressed in glutamate-ergic and GABA-ergic neurons in the visual cortex of adult mice [41]. The primary structure of Lypd6b is characterized by a high degree of homology with Lypd6 (~54%; Fig. 1). Similarly to Lypd6, Lypd6b has in its structure a C-terminal amino acid sequence to which a GPI anchor can potentially attach, but presence of a GPI anchor has been experimentally confirmed only for Lypd6 [2]. Unlike other Ly6/uPAR proteins, Lypd6 and Lypd6b besides classical three-finger domain [64] have unusual additional extended N- and C-terminal sequences adjacent to the LU domain (Fig. 1). The role of these extended sequences is still unknown. Co-expression of Lypd6b with α3β4-nAChR increases the desensitization rate and the sensitivity of (α3)3(β4)2 nAChRs to ACh in Xenopus oocytes. Moreover, Lypd6b inhibits α3α5Dβ4-nAChRs, but it does not affect α3α5Nβ4 receptors, which are distinguished by the replacement of D398N in the α5 subunit associated with the development of nicotine addiction [65].
PSCA, a prostate stem cell antigen. PSCA exists in membrane-associated and soluble forms [66, 67]. PSCA is expressed in various organs and tissues, such as bladder, kidney, esophagus, stomach, skin, prostate basal cells, and placenta tissues [68]. PSCA is a marker for some tumors – prostate, stomach, and bladder [69]. PSCA demonstrates an oncogenic activity in prostate cancer and glioma, but PSCA suppresses cell growth in gastric and gallbladder cancers [68].
PSCA expression was found in the chicken ciliary ganglion neurons on the 14th day of embryonic development. In the late stages of development of chicken embryos, low psca expression was also observed in the pectoral muscles, liver, ovaries, testicles, heart, and cerebellum. Significantly higher levels of psca were found in the telencephalon and peripheral nervous system [5]. Neuronal tissue of adult mice, as in the case of chicken embryos, contains much more psca than nonneuronal tissues. Moreover, correlation of psca expression with expression of α7-nAChRs was found [5].
PSCA blocks activation of α7-nAChRs in the ciliary ganglion neurons and rescues neurons from cell death, and overexpression of psca in chicken embryos leads to a decrease in the death of the choroid neurons, but not ciliary ganglion neurons [5]. PSCA inhibits nicotine-induced phosphorylation of MAP kinase ERK1/2 in PC12 cells, possibly thus regulating synaptic plasticity [67]. The levels of PSCA were increased by approximately 70% in the frontal cortex of patients with Alzheimer’s disease compared with healthy people. This probably indicates involvement of PSCA in the pathogenesis of Alzheimer’s disease [67].
Secreted proteins SLURP-1 and SLURP-2. SLURP-1 and SLURP-2 are found in epithelial and immune cells of mammals and are considered as auto/paracrine regulators of epithelial homeostasis [70]. SLURP proteins control the growth, migration, and differentiation of epithelial cells, as well as the development of inflammation and tumors [70]. SLURP-1 has antiproliferative activity and promotes apoptosis of human keratinocytes [71], while SLURP-2 accelerates cell growth, decreasing their differentiation and depressing response to proapoptotic signals [70]. It has been shown that SLURP proteins regulate skin and mucous wound healing [72] and participate in protecting skin cells from oncogenic transformation caused by nitrosamines [70, 71]. Point mutations in the slurp-1 gene cause an autosomal inflammatory disease of the skin and nails called Mal de Meleda [73]. Inhibition of the slurp-2 gene results in development of palmar-plantar keratoderma [74], and the slurp-2 expression level is increased in psoriasis patients [75]. SLURP-1 expression was detected in human HT-29 colorectal adenocarcinoma cells, and the level of SLURP-1 expression in these cells is significantly reduced by nicotine treatment [76]. Incubation of HT-29 cells with recombinant proteins SLURP-1 and SLURP-2 significantly inhibits cell growth [77]. SLURP proteins may also be involved in the work of the immune system [78] and are expressed in sensory neurons [79].
SLURP-1 selectively interacts with α7-nAChRs and inhibits the ACh-induced current on α7-nAChR expressed in Xenopus oocytes, with IC50 ~ 1 µM. Besides, SLURP-1 does not compete with 125I-labeled α-Bgtx for interaction with α7-nAChR, indicating that SLURP-1 binds not to the orthosteric binding site of the receptor [62]. The antiproliferative activity of SLURP-1 is probably associated with its interaction with α7-nAChR. It is assumed that the interaction of SLURP-1 with α7-nAChR triggers intracellular processes associated with both Ca2+ current through the receptor channel and activation of kinases by a metabotropic pathway [80]. This is confirmed by the lack of influence of α-Bgtx on the antiproliferative activity of SLURP-1 [62].
The structure of SLURP-1 is unusual for three-finger proteins: there is a well-structured core, but none of the three loops of the molecule forms an ordered structure (Fig. 11). This, together with the cis-trans isomerization of the Tyr39–Pro40 peptide bond in the central loop, causes the unusual conformational plasticity of the loops in solution. Such flexible structure indicates the possibility that SLURP-1 binds to different targets associated with signal transmission by ionotropic and metabotropic pathways.
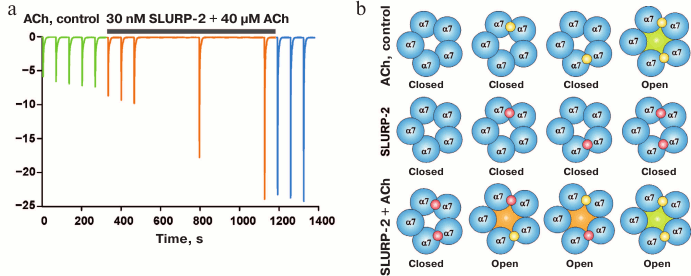
Until recently, it was believed that the auto/paracrine activity of SLURP-2 was mediated by its selective interaction with α3β2- and α9-nAChR [81]. However, in 2016 it was shown that SLURP-2 could extract from the cerebral cortex the α3, α4, α5, α7, β2, and β4 nAChR subunits, indicating its wider pharmacological profile [82]. It was shown that SLURP-2 inhibits ACh-induced currents through α4β2- and α3β2-nAChRs expressed in Xenopus oocytes [82]. In the case of α7-nAChRs, SLURP-2, like Lynx1, at concentrations of ≤1 µM significantly increases the ACh-evoked current, but inhibits the receptor at higher concentrations [82]. Under certain conditions, SLURP-2 could increase the current amplitude through α7-nAChR more than 5-fold (Fig. 12). The observed effect of receptor activation at low ligand concentrations recalls the “priming effect” described for other nAChR ligands, such as tubocurarine [83]. In addition, it was reported that SLURP-2 could allosterically modulate human M1 and M3 mAChRs [82]. The interaction of SLURP-2 with α3β2-nAChR and M3-mAChR increases human keratinocyte proliferation and interaction with α7-nAChR leads to inhibition of cell growth [82]. It is known that keratinocytes express various types of acetylcholine receptors at different stages of maturation, so the effects of SLURP-2 could depend on the stage of cell development.
Fig. 12. “Priming” effect of SLURP-2 on α7-nAChR expressed in X. laevis oocytes. a) Electrophysiological records of ACh-induced current in absence and presence of 30 nM SLURP-2. Currents were elicited by 5 s pulses of 40 µM ACh. A representative trace from nine independent experiments is shown. Green traces represent the responses evoked by ACh in absence of the compound, orange traces are the responses evoked by the same ACh test pulse in presence of SLURP-2, and blue traces are the responses evoked by ACh after terminating the SLURP-2 application. b) A diagram explaining the “priming” effect of SLURP-2 on α7-nAChR. The ACh and SLURP-2 molecules are indicated by yellow and red balls, respectively. The figure was adapted from [82].
As in the case of WTX, Lynx1, and SLURP-1, the SLURP-2 loops exhibit significant conformational plasticity due to motions in the ps-ns time range (Fig. 11) [82]. Interestingly, Lynx1 and SLURP-2 are the products of one gene, but the proteins have a low degree of homology due to an alternative splicing (~30%). Nevertheless, analysis of the pharmacological and structural properties of the Lynx1 and SLURP-2 molecules indicates great functional similarity of these proteins.
More than 40 years ago, three-finger proteins, having structural homology with snake α-neurotoxins and often similar molecular targets, for example, nicotinic acetylcholine receptors, were found in mammals. This raised many questions regarding evolutionary relationships in the Ly6/uPAR family. Today, many of these questions remain open. The detection of three-finger proteins in insects, fish, and amphibians indicates that snake neurotoxins have later evolutionary origin and probably have been “developed” by nature based on endogenous regulators of the vital receptors [4]. Apparently, in the course of evolution, neurotoxins accumulated their unique properties, namely, high selectivity, affinity, and the ability to block target receptors irreversibly.
All known to date endogenous members of the Ly6/uPAR family play crucial roles in the functioning of the organism. Violation of their expression leads to the development of various diseases or is lethal. Recent advances in genome sequencing and proteome analysis give a hope for the discovery of new representatives of the three-finger protein family in various organisms. Therefore, the creation of effective recombinant systems for the Ly6/uPAR protein production making it possible to obtain milligram quantities of the proteins for structural and functional studies is especially important [84].
Acknowledgments
This work was supported by the Russian Science Foundation (project No. 16-14-00102).
REFERENCES
1.Koh, K., Joiner, W. J., Wu, M. N., Yue, Z., Smith,
C. J., and Sehgal, A. (2008) Identification of SLEEPLESS, a
sleep-promoting factor, Science, 321, 372-376.
2.Ozhan, G., Sezgin, E., Wehner, D., Pfister, A. S.,
Kuhl, S. J., Kagermeier-Schenk, B., Kuhl, M., Schwille, P., and
Weidinger, G. (2013) Lypd6 enhances Wnt/β-catenin signaling by
promoting Lrp6 phosphorylation in raft plasma membrane domains, Dev.
Cell, 26, 331-345.
3.Da Silva, S. M., Gates, P. B., and Brockes, J. P.
(2002) The newt ortholog of CD59 is implicated in proximodistal
identity during amphibian limb regeneration, Dev. Cell,
3, 547-555.
4.Fry, B. G., Wuster, W., Kini, R. M., Brusic, V.,
Khan, A., Venkataraman, D., and Rooney, A. P. (2003) Molecular
evolution and phylogeny of elapid snake venom three-finger toxins,
J. Mol. Evol., 57, 110-129.
5.Hruska, M., Keefe, J., Wert, D., Tekinay, A. B.,
Hulce, J. J., Ibanez-Tallon, I., and Nishi, R. (2009) Prostate stem
cell antigen is an endogenous lynx1-like prototoxin that antagonizes
alpha7-containing nicotinic receptors and prevents programmed cell
death of parasympathetic neurons, J. Neurosci., 29,
14847-14854.
6.Miwa, J. M., Ibanez-Tallon, I., Crabtree, G. W.,
Sanchez, R., Sali, A., Role, L. W., and Heintz, N. (1999) Lynx1, an
endogenous toxin-like modulator of nicotinic acetylcholine receptors in
the mammalian CNS, Neuron, 23, 105-114.
7.Kini, R. M., and Doley, R. (2010) Structure,
function and evolution of three-finger toxins: mini proteins with
multiple targets, Toxicon, 56, 855-867.
8.Loughner, C. L., Bruford, E. A., McAndrews, M. S.,
Delp, E. E., Swamynathan, S., and Swamynathan, S. K. (2016)
Organization, evolution and functions of the human and mouse Ly6/uPAR
family genes, Hum. Genom., 10, 10.
9.Tsetlin, V. I., and Hucho, F. (2004) Snake and
snail toxins acting on nicotinic acetylcholine receptors: fundamental
aspects and medical applications, FEBS Lett., 557,
9-13.
10.Lyukmanova, E. N., Shenkarev, Z. O., Schulga, A.
A., Ermolyuk, Y. S., Mordvintsev, D. Y., Utkin, Y. N., Shoulepko, M.
A., Hogg, R. C., Bertrand, D., Dolgikh, D. A., Tsetlin, V. I., and
Kirpichnikov, M. P. (2007) Bacterial expression, NMR, and
electrophysiology analysis of chimeric short/long-chain
alpha-neurotoxins acting on neuronal nicotinic receptors, J. Biol.
Chem., 282, 24784-24791.
11.Huang, S., Li, S. X., Bren, J., Cheng, K.,
Gomoto, R., Chen, L., and Sine, S. M. (2013) Complex between
α-bungarotoxin and an α7 nicotinic receptor ligand-binding
domain chimaera, Biochem. J., 454, 303-310.
12.Servent, D., Blanchet, G., Mourier, G., Marquer,
C., Marcon, E., and Fruchart-Gaillard, C. (2011) Muscarinic toxins,
Toxicon, 58, 455-463.
13.Marquer, C., Fruchart-Gaillard, C., Letellier,
G., Marcon, E., Mourier, G., Zinn-Justin, S., Menez, A., Servent, D.,
and Gilquin, B. (2011) Structural model of ligand-G protein-coupled
receptor (GPCR) complex based on experimental double mutant cycle data:
MT7 snake toxin bound to dimeric hM1 muscarinic receptor, J. Biol.
Chem., 286, 31661-31675.
14.Nirthanan, S., Gopalakrishnakone, P., Gwee, M.
C., Khoo, H. E., and Kini, R. M. (2003) Non-conventional toxins from
Elapid venoms, Toxicon, 41, 397-407.
15.Mordvintsev, D. Y., Polyak, Y. L., Rodionov, D.
I., Jakubik, J., Dolezal, V., Karlsson, E., Tsetlin, V. I., and Utkin,
Y. N. (2009) Weak toxin WTX from Naja kaouthia cobra venom
interacts with both nicotinic and muscarinic acetylcholine receptors,
FEBS J., 276, 5065-5075.
16.Lyukmanova, E. N., Shulepko, M. A., Shenkarev, Z.
O., Kasheverov, I. E., Chugunov, A. O., Kulbatskii, D. S., Myshkin, M.
Y., Utkin, Y. N., Efremov, R. G., Tsetlin, V. I., Arseniev, A. S.,
Kirpichnikov, M. P., and Dolgikh, D. A. (2016) Central loop of
non-conventional toxin WTX from Naja kaouthia is important for
interaction with nicotinic acetylcholine receptors, Toxicon,
119, 274-279.
17.Lyukmanova, E. N., Shenkarev, Z. O., Shulepko, M.
A., Paramonov, A. S., Chugunov, A. O., Janickova, H., Dolejsi, E.,
Dolezal, V., Utkin, Y. N., Tsetlin, V. I., Arseniev, A. S., Efremov, R.
G., Dolgikh, D. A., and Kirpichnikov, M. P. (2015) Structural insight
into specificity of interactions between nonconventional three-finger
weak toxin from Naja kaouthia (WTX) and muscarinic
acetylcholine receptors, J. Biol Chem., 290,
23616-23630.
18.Rosso, J. P., Schwarz, J. R., Diaz-Bustamante,
M., Ceard, B., Gutierrez, J. M., Kneussel, M., Pongs, O., Bosmans, F.,
and Bougis, P. E. (2015) MmTX1 and MmTX2 from coral snake venom
potently modulate GABAA receptor activity, Proc. Natl.
Acad. Sci. USA, 112, E891-900.
19.Hannan, S., Mortensen, M., and Smart, T. G.
(2015) Snake neurotoxin α-bungarotoxin is an antagonist at native
GABA(A) receptors, Neuropharmacology, 93, 28-40.
20.Kudryavtsev, D. S., Shelukhina, I. V., Son, L.
V., Ojomoko, L. O., Kryukova, E. V., Lyukmanova, E. N., Zhmak, M. N.,
Dolgikh, D. A., Ivanov, I. A., Kasheverov, I. E., Starkov, V. G.,
Ramerstorfer, J., Sieghart, W., Tsetlin, V. I., and Utkin, Y. N. (2015)
Neurotoxins from snake venoms and α-conotoxin ImI inhibit
functionally active ionotropic γ-aminobutyric acid (GABA)
receptors, J. Biol. Chem., 290, 22747-22758.
21.Diochot, S., Baron, A., Salinas, M., Douguet, D.,
Scarzello, S., Dabert-Gay, A. S., Debayle, D., Friend, V., Alloui, A.,
Lazdunski, M., and Lingueglia, E. (2012) Black mamba venom peptides
target acid-sensing ion channels to abolish pain, Nature,
490, 552-555.
22.Mourier, G., Salinas, M., Kessler, P., Stura, E.
A., Leblanc, M., Tepshi, L., Besson, T., Diochot, S., Baron, A.,
Douguet, D., Lingueglia, E., and Servent, D. (2016) Mambalgin-1
pain-relieving peptide, stepwise solid-phase synthesis, crystal
structure, and functional domain for acid-sensing ion channel 1a
inhibition, J. Biol. Chem., 291, 2616-2629.
23.Efremov, R. G., Volynsky, P. E., Nolde, D. E.,
Dubovskii, P. V., and Arseniev, A. S. (2002) Interaction of
cardiotoxins with membranes: a molecular modeling study, Biophys.
J., 83, 144-153.
24.Dubovskii, P. V., Konshina, A. G., and Efremov,
R. G. (2014) Cobra cardiotoxins: membrane interactions and
pharmacological potential, Curr. Med. Chem., 21,
270-287.
25.Feofanov, A. V., Sharonov, G. V., Astapova, M.
V., Rodionov, D. I., Utkin, Y. N., and Arseniev, A. S. (2005) Cancer
cell injury by cytotoxins from cobra venom is mediated through
lysosomal damage, Biochem. J., 390, 11-18.
26.Cervenansky, C., Dajas, F., Harvey, A. L., and
Karlsson, E. (1991) in International Encyclopedia of Pharmacology
and Therapeutics: Snake Toxins (Harvey, A. L., ed.) Pergamon
Press, New York, pp. 303-321.
27.Bourne, Y., Taylor, P., and Marchot, P. (1995)
Acetylcholinesterase inhibition by fasciculin: crystal structure of the
complex, Cell, 83, 503-512.
28.Wu, M., Robinson, J. E., and Joiner, W. J. (2014)
SLEEPLESS is a bifunctional regulator of excitability and cholinergic
synaptic transmission, Curr. Biol., 24, 621-629.
29.Wu, M., Liu, C. Z., and Joiner, W. J. (2016)
Structural analysis and deletion mutagenesis define regions of
QUIVER/SLEEPLESS that are responsible for interactions with shaker-type
potassium channels and nicotinic acetylcholine receptors, PLoS
One, 11, e0148215.
30.Yang, B., Yao, X., Gu, S., Zhang, Y., Liu, Z.,
and Zhang, Y. (2010) Selectivity of lynx proteins on insect nicotinic
acetylcholine receptors in the brown planthopper, Nilaparvata
lugens, Insect Mol. Biol., 19, 283-289.
31.McNally, J. D., Wu, S. B., Sturgeon, C. M., and
Storey, K. B. (2002) Identification and characterization of a novel
freezing inducible gene, li16, in the wood frog Rana
sylvatica, FASEB J., 16, 902-904.
32.Kumar, A., Gates, P. B., Czarkwiani, A., and
Brockes, J. P. (2015) An orphan gene is necessary for preaxial digit
formation during salamander limb development, Nat. Commun.,
6, 8684.
33.Nomura, K., Tanimoto, Y., Hayashi, F., Harada,
E., Shan, X. Y., Shionyu, M., Hijikata, A., Shirai, T., Morigaki, K.,
and Shimamoto, K. (2017) The role of the Prod1 membrane anchor in newt
limb regeneration, Angew. Chem. Int. Ed. Engl., 56,
270-274.
34.Wang, M., Li, L., Guo, Q., Zhang, S., Ji, D., and
Li, H. (2016) Identification and expression of a new Ly6 gene
cluster in zebrafish Danio rerio, with implications of
being involved in embryonic immunity, Fish Shellfish Immunol.,
54, 230-240.
35.Fletcher, C. M., Harrison, R. A., Lachmann, P.
J., and Neuhaus, D. (1994) Structure of a soluble, glycosylated form of
the human complement regulatory protein CD59, Structure,
2, 185-199.
36.Parker, C., Omine, M., Richards, S., Nishimura,
J., Bessler, M., Ware, R., Hillmen, P., Luzzatto, L., Young, N.,
Kinoshita, T., Rosse, W., and Socie, G. (2005) Diagnosis and management
of paroxysmal nocturnal hemoglobinuria, Blood, 106,
3699-3709.
37.Blasi, F., and Carmeliet, P. (2002) uPAR: a
versatile signaling orchestrator, Nat. Rev. Mol. Cell Biol.,
3, 932-943.
38.Su, S. C., Lin, C. W., Yang, W. E., Fan, W. L.,
and Yang, S. F. (2016) The urokinase-type plasminogen activator (uPA)
system as a biomarker and therapeutic target in human malignancies,
Expert Opin. Ther. Targets, 20, 551-566.
39.Huai, Q., Mazar, A. P., Kuo, A., Parry, G. C.,
Shaw, D. E., Callahan, J., Li, Y., Yuan, C., Bian, C., Chen, L., Furie,
B., Furie, B. C., Cines, D. B., and Huang, M. (2006) Structure of human
urokinase plasminogen activator in complex with its receptor,
Science, 311, 656-659.
40.Ibanez-Tallon, I., Miwa, J. M., Wang, H. L.,
Adams, N. C., Crabtree, G. W., Sine, S. M., and Heintz, N. (2002) Novel
modulation of neuronal nicotinic acetylcholine receptors by association
with the endogenous prototoxin lynx1, Neuron, 33,
893-903.
41.Demars, M. P., and Morishita, H. (2014) Cortical
parvalbumin and somatostatin GABA neurons express distinct endogenous
modulators of nicotinic acetylcholine receptors, Mol. Brain,
7, 75-79.
42.Nichols, W. A., Henderson, B. J., Yu, C., Parker,
R. L., Richards, C. I., Lester, H. A., and Miwa, J. M. (2014) Lynx1
shifts α4β2 nicotinic receptor subunit stoichiometry by
affecting assembly in the endoplasmic reticulum, J. Biol. Chem.,
289, 31423-31432.
43.Miwa, J. M., Stevens, T. R., King, S. L.,
Caldarone, B. J., Ibanez-Tallon, I., Xiao, C., Fitzsimonds, R. M.,
Pavlides, C., Lester, H. A., Picciotto, M. R., and Heintz, N. (2006)
The prototoxin lynx1 acts on nicotinic acetylcholine receptors to
balance neuronal activity and survival in vivo, Neuron,
51, 587-600.
44.Miwa, J. M., and Walz, A. (2012) Enhancement in
motor learning through genetic manipulation of the Lynx1 gene,
PLoS One, 7, e43302.
45.Kobayashi, A., Parker, R. L., Wright, A. P.,
Brahem, H., Ku, P., Oliver, K. M., Walz, A., Lester, H. A., and Miwa,
J. M. (2014) Lynx1 supports neuronal health in the mouse dorsal
striatum during aging: an ultrastructural investigation, J. Mol.
Neurosci., 53, 525-536.
46.Morishita, H., Miwa, J. M., Heintz, N., and
Hensch, T. K. (2010) Lynx1, a cholinergic brake, limits plasticity in
adult visual cortex, Science, 330, 1238-1240.
47.Bukhari, N., Burman, P. N., Hussein, A., Demars,
M. P., Sadahiro, M., Brady, D. M., Tsirka, S. E., Russo, S., and
Morishita, H. (2015) Unmasking proteolytic activity for adult visual
cortex plasticity by the removal of Lynx1, J. Neurosci.,
35, 12693-12702.
48.Sajo, M., Ellis-Davies, G., and Morishita, H.
(2016) Lynx1 limits dendritic spine turnover in the adult visual
cortex, J. Neurosci., 36, 9472-9478.
49.Shulepko, M. A., Lyukmanova, E. N., Kasheverov,
I. E., Dolgikh, D. A., Tsetlin, V. I., and Kirpichnikov, M. P. (2011)
Bacterial expression of the water-soluble domain of lynx1, an
endogenous neuromodulator of human nicotinic receptors, Russ. J.
Bioorg. Chem., 37, 609-615.
50.Lyukmanova, E. N., Shenkarev, Z. O., Shulepko, M.
A., Mineev, K. S., D'Hoedt, D., Kasheverov, I. E., Filkin, S. Y.,
Krivolapova, A. P., Janickova, H., Dolezal, V., Dolgikh, D. A.,
Arseniev, A. S., Bertrand, D., Tsetlin, V. I., and Kirpichnikov, M. P.
(2011) NMR structure and action on nicotinic acetylcholine receptors of
water-soluble domain of human LYNX1, J. Biol. Chem., 286,
10618-10627.
51.Lyukmanova, E. N., Shulepko, M. A., Buldakova, S.
L., Kasheverov, I. E., Shenkarev, Z. O., Reshetnikov, R. V., Filkin, S.
Y., Kudryavtsev, D. S., Ojomoko, L. O., Kryukova, E. V., Dolgikh, D.
A., Kirpichnikov, M. P., Bregestovski, P. D., and Tsetlin, V. I. (2013)
Ws-LYNX1 residues important for interaction with muscle-type and/or
neuronal nicotinic receptors, J. Biol. Chem., 288,
15888-15899.
52.Taly, A., Corringer, P. J., Guedin, D., Lestage,
P., and Changeux, J. P. (2009) Nicotinic receptors: allosteric
transitions and therapeutic targets in the nervous system, Nat. Rev.
Drug Discov., 8, 733-750.
53.Thomsen, M. S., Cinar, B., Jensen, M. M.,
Lyukmanova, E. N., Shulepko, M. A., Tsetlin, V., Klein, A. B., and
Mikkelsen, J. D. (2014) Expression of the Ly-6 family proteins Lynx1
and Ly6H in the rat brain is compartmentalized, cell-type specific, and
developmentally regulated, Brain Struct. Funct., 219,
1923-1934.
54.Thomsen, M. S., Arvaniti, M., Jensen, M. M.,
Shulepko, M. A., Dolgikh, D. A., Pinborg, L. H., Hartig, W.,
Lyukmanova, E. N., and Mikkelsen, J. D. (2016) Lynx1 and Aβ1-42
bind competitively to multiple nicotinic acetylcholine receptor
subtypes, Neurobiol. Aging, 46, 13-21.
55.Fu, X. W., Song, P. F., and Spindel, E. R. (2015)
Role of Lynx1 and related Ly6 proteins as modulators of cholinergic
signaling in normal and neoplastic bronchial epithelium, Int.
Immunopharmacol., 29, 93-98.
56.Dessaud, E., Salaun, D., Gayet, O., Chabbert, M.,
and De Lapeyriere, O. (2006) Identification of lynx2, a novel member of
the ly-6/neurotoxin superfamily, expressed in neuronal subpopulations
during mouse development, Mol. Cell Neurosci., 31,
232-242.
57.Tekinay, A. B., Nong, Y., Miwa, J. M., Lieberam,
I., Ibanez-Tallon, I., Greengard, P., and Heintz, N. (2009) A role for
LYNX2 in anxiety-related behavior, Proc. Natl. Acad. Sci. USA,
106, 4477-4482.
58.Wu, M., Puddifoot, C. A., Taylor, P., and Joiner,
W. J. (2015) Mechanisms of inhibition and potentiation of
α4β2 nicotinic acetylcholine receptors by members of the Ly6
protein family, J. Biol. Chem., 290, 24509-24518.
59.Darvas, M., Morsch, M., Racz, I., Ahmadi, S.,
Swandulla, D., and Zimmer, A. (2009) Modulation of the Ca2+
conductance of nicotinic acetylcholine receptors by Lypd6, Eur.
Neuropsychopharmacol., 19, 670-681.
60.Arvaniti, M., Jensen, M. M., Soni, N., Wang, H.,
Klein, A. B., Thiriet, N., Pinborg, L. H., Muldoon, P. P., Wienecke,
J., Imad Damaj, M., Kohlmeier, K. A., Gondre-Lewis, M. C., Mikkelsen,
J. D., and Thomsen, M. S. (2016) Functional interaction between Lypd6
and nicotinic acetylcholine receptors, J. Neurochem.,
138, 806-820.
61.Zhang, Y., Lang, Q., Li, J., Xie, F., Wan, B.,
and Yu, L. (2010) Identification and characterization of human LYPD6, a
new member of the Ly-6 superfamily, Mol. Biol. Rep., 37,
2055-2062.
62.Lyukmanova, E. N., Shulepko, M. A., Kudryavtsev,
D., Bychkov, M. L., Kulbatskii, D. S., Kasheverov, I. E., Astapova, M.
V., Feofanov, A. V., Thomsen, M. S., Mikkelsen, J. D., Shenkarev, Z.
O., Tsetlin, V. I., Dolgikh, D. A., and Kirpichnikov M. P. (2016) Human
secreted Ly-6/uPAR related protein-1 (SLURP-1) is a selective
allosteric antagonist of α7 nicotinic acetylcholine receptor,
PLoS One, 11, e0149733.
63.Ni, J., Lang, Q., Bai, M., Zhong, C., Chen, X.,
Wan, B., and Yu, L. (2009) Cloning and characterization of a human
LYPD7, a new member of the Ly-6 superfamily, Mol. Biol. Rep.,
36, 697-703.
64.Paramonov, A. S., Kulbatskii, D. S., Loktyushov,
E. V., Tsarev, A. V., Dolgikh, D. A., Shenkarev, Z. O., Kirpichnikov,
M. P., and Lyukmanova, E. N. (2017) Recombinant production and
structural study of the human Lypd6 and Lypd6B proteins, Russ. J.
Bioorg. Chem., 43, 644-652.
65.Ochoa, V., George, A. A., Nishi, R., and
Whiteaker, P. (2016) The prototoxin LYPD6B modulates heteromeric
α3β4-containing nicotinic acetylcholine receptors, but not
α7 homomers, FASEB J., 30, 809-816.
66.Reiter, R. E., Gu, Z., Watabe, T., Thomas, G.,
Szigeti, K., Davis, E., Wahl, M., Nisitani, S., Yamashiro, J., Le Beau,
M. M., Loda, M., and Witte, O. N. (1998) Prostate stem cell antigen: a
cell surface marker overexpressed in prostate cancer, Proc. Natl.
Acad. Sci. USA, 95, 1735-1740.
67.Jensen, M. M., Arvaniti, M., Mikkelsen, J. D.,
Michalski, D., Pinborg, L. H., Hartig, W., and Thomsen, M. S. (2015)
Prostate stem cell antigen interacts with nicotinic acetylcholine
receptors and is affected in Alzheimer’s disease, Neurobiol.
Aging, 36, 1629-1638.
68.Ono, H., Hiraoka, N., Lee, Y. S., Woo, S. M.,
Lee, W. J., Choi, I. J., Saito, A., Yanagihara, K., Kanai, Y., Ohnami,
S., Chiwaki, F., Sasaki, H., Sakamoto, H., Yoshida, T., and Saeki, N.
(2012) Prostate stem cell antigen, a presumable organ-dependent tumor
suppressor gene, is down-regulated in gallbladder carcinogenesis,
Genes Chromosomes Cancer, 51, 30-41.
69.Moore, M. L., Teitell, M. A., Kim, Y., Watabe,
T., Reiter, R. E., Witte, O. N., and Dubey, P. (2008) Deletion of PSCA
increases metastasis of TRAMP-induced prostate tumors without altering
primary tumor formation, Prostate, 68, 139-151.
70.Arredondo, J., Chernyavsky, A. I., and Grando, S.
A. (2007) SLURP-1 and -2 in normal, immortalized and malignant oral
keratinocytes, Life Sci., 80, 2243-2247.
71.Arredondo, J., Chernyavsky, A. I., Webber, R. J.,
and Grando, S. A. (2005) Biological effects of SLURP-1 on human
keratinocytes, J. Invest. Dermatol., 125, 1236-1241.
72.Chernyavsky, A. I., Kalantari-Dehaghi, M.,
Phillips, C., Marchenko, S., and Grando, S. A. (2012) Novel cholinergic
peptides SLURP-1 and -2 regulate epithelialization of cutaneous and
oral wounds, Wound Rep. Regen., 20, 103-113.
73.Perez, C., and Khachemoune, A. (2016) Mal de
Meleda: a focused review, Am. J. Clin. Dermatol., 17,
63-70.
74.Allan, C. M., Procaccia, S., Tran, D., Tu, Y.,
Barnes, R. H., 2nd, Larsson, M., Allan, B. B., Young, L. C., Hong, C.,
Tontonoz, P., Fong, L. G., Young, S. G., and Beigneux, A. P. (2016)
Palmoplantar keratoderma in Slurp2-deficient mice, J. Invest.
Dermatol., 136, 436-443.
75.Tsuji, H., Okamoto, K., Matsuzaka, Y., Iizuka,
H., Tamiya, G., and Inoko, H. (2003) SLURP-2, a novel member of the
human Ly-6 superfamily that is up-regulated in psoriasis vulgaris,
Genomics, 81, 26-33.
76.Pettersson, A., Nylund, G., Khorram-Manesh, A.,
Nordgren, S., and Delbro, D. S. (2009) Nicotine induced modulation of
SLURP-1 expression in human colon cancer cells, Auton.
Neurosci., 148, 97-100.
77.Lyukmanova, E. N., Shulepko, M. A., Bychkov, M.
L., Shenkarev, Z. O., Paramonov, A. S., Chugunov, A. O., Arseniev, A.
S., Dolgikh, D. A., and Kirpichnikov, M. P. (2014) Human SLURP-1 and
SLURP-2 proteins acting on nicotinic acetylcholine receptors reduce
proliferation of human colorectal adenocarcinoma HT-29 cells, Acta
Naturae, 6, 60-66.
78.Moriwaki, Y., Yoshikawa, K., Fukuda, H., Fujii,
Y. X., Misawa, H., and Kawashima, K. (2007) Immune system expression of
SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor
ligands, Life Sci., 80, 2365-2368.
79.Moriwaki, Y., Watanabe, Y., Shinagawa, T., Kai,
M., Miyazawa, M., Okuda, T., Kawashima, K., Yabashi, A., Waguri, S.,
and Misawa, H. (2009) Primary sensory neuronal expression of SLURP-1,
an endogenous nicotinic acetylcholine receptor ligand, Neurosci.
Res., 64, 403-412.
80.Chernyavsky, A. I., Arredondo, J., Galitovskiy,
V., Qian, J., and Grando, S. A. (2010) Upregulation of nuclear
factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic
acetylcholine receptor and involves both ionic events and activation of
protein kinases, Am. J. Physiol. Cell Physiol., 299,
903-911.
81.Arredondo, J., Chernyavsky, A. I., Jolkovsky, D.
L., Webber, R. J., and Grando, S. A. (2006) SLURP-2: a novel
cholinergic signaling peptide in human mucocutaneous epithelium, J.
Cell. Physiol., 208, 238-245.
82.Lyukmanova, E. N., Shulepko, M. A., Shenkarev, Z.
O., Bychkov, M. L., Paramonov, A. S., Chugunov, A. O., Kulbatskii, D.
S., Arvaniti, M., Dolejsi, E., Schaer, T., Arseniev, A. S., Efremov, R.
G., Thomsen, M. S., Dolezal, V., Bertrand, D., Dolgikh, D. A., and
Kirpichnikov, M. P. (2016) Secreted isoform of human Lynx1 (SLURP-2):
spatial structure and pharmacology of interactions with different types
of acetylcholine receptors, Sci. Rep., 6, 30698.
83.Cachelin, A. B., and Rust, G. (1994) Unusual
pharmacology of (+)-tubocurarine with rat neuronal nicotinic
acetylcholine receptors containing beta 4 subunits, Mol.
Pharmacol., 46, 1168-1174.
84.Shulepko, M. A., Lyukmanova, E. N., Shenkarev, Z.
O., Dubovskii, P. V., Astapova, M. V., Feofanov, A. V., Arseniev, A.
S., Utkin, Y. N., Kirpichnikov, M. P., and Dolgikh, D. A. (2017)
Towards universal approach for bacterial production of three-finger
Ly6/uPAR proteins: case study of cytotoxin I from cobra N.
oxiana, Protein Expr. Purif., 130, 13-20.