Biotransformation of Progesterone by the Ascomycete Aspergillus niger N402
O. S. Savinova1*, P. N. Solyev2, D. V. Vasina1, T. V. Tyazhelova1, T. V. Fedorova1, and T. S. Savinova3
1Bach Institute of Biochemistry, Biotechnology Research Center, Russian Academy of Sciences, 119071 Moscow, Russia; E-mail: savinova_os@rambler.ru2Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia
3Lomonosov Moscow State University, Faculty of Chemistry, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received August 4, 2017; Revision received September 15, 2017
The ability of the ascomycete Aspergillus niger N402 to transform exogenous progesterone was investigated. We found that this strain has steroid-hydroxylating activity and can introduce a hydroxyl group into the progesterone molecule mainly at positions C11(α) and C21 with predominant formation of 21-hydroxyprogesterone (deoxycortone). In addition, formation of 6β,11α-dihydroxyprogesterone was also observed. Studying the effects of the growth medium composition and temperature on progesterone conversion by A. niger N402 showed that the most intense accumulation of 21-hydroxyprogesterone occurred in minimal synthetic medium at 28°C. Increasing the cultivation temperature to 37°C resulted in almost complete inhibition of the hydroxylase activity in the minimal medium. In the complete medium, a similar increase in temperature inhibited 11α-hydroxylase activity and completely suppressed 6β-hydroxylase activity, but it produced no effect on 21-hydroxylating activity.
KEY WORDS: Aspergillus niger N402, progesterone, biotransformation, 21-hydroxylation, 11α-hydroxylation, deoxycortoneDOI: 10.1134/S0006297918010030
Abbreviations: CM, complete medium; HRMS, high-resolution mass spectrometry; MM, minimal medium; NMR, nuclear magnetic resonance; TLC, thin-layer chromatography.
Highly selective one-step hydroxylation of steroids can be achieved only
with enzymatic systems of microorganisms, mostly mycelial fungi,
including those from the genus Aspergillus. Almost all
Aspergillus fungi convert progesterone into its
11α-derivative. However, some Aspergillus strains can
introduce hydroxyl groups at other positions (i.e. position 21) of the
progesterone molecule. Since 21-hydroxyprogesterone is a commonly used
drug, the reaction of progesterone hydroxylation at position 21 is of
considerable practical interest. 21-Hydroxyprogesterone
(deoxycorticosterone, INN desoxycortone, CAS No. 64-85-7) is a natural
hormone with mineralocorticoid activity that is produced by the adrenal
cortex. It is used in medicine in the form of its esters (acetate,
pivalate) for treatment of Addison’s disease, hypocorticism,
myasthenia, adynamia, and other disorders [1]. The
use of chemical methods for hydroxyl group introduction at position 21
is hindered by several undesirable side reactions [2]. Because of this, the search for new strains
capable of selective biological transformation of progesterone into
21-hydroxyprogesterone is extremely important.
The process of targeted progesterone hydroxylation strongly depends on the transforming strain, but also on the cultivation conditions, such as medium composition and pH, presence of metal ions, and temperature regime. El-Kady [3] demonstrated that Aspergillus niger cultures hydroxylate progesterone at positions 6β and 11α. When 11α-hydroxyprogesterone was used as a substrate, the 6β-hydroxylase activity achieved a maximum at pH 6.5 and was inhibited by Co2+ and Cd2+. The ability of A. niger to transform progesterone was also found by Fouad et al. [4], who showed that A. niger converted progesterone into a mixture of 11α-hydroxyprogesterone and 6β,11α-dihydroxyprogesterone at a 65.7 : 35.5 ratio. Metwali [5] showed that A. niger (isolate No. 11/3) transformed progesterone into 21-hydroxyprogesterone, and this reaction was significantly affected by the medium composition and cultivation conditions of (pH and temperature) – the maximal 21-hydroxyprogesterone formation being observed after 48 h of transformation at 28°C and pH 6.5.
In microorganisms, hydroxylation of steroids is catalyzed by cytochrome P450-dependent monooxygenases [6]. So far, no data have been published on the ability of cytochrome P450-dependent monooxygenases from the A. niger N402 to introduce a hydroxyl group into an exogenous steroid molecule, so we investigated the capacity of this strain to biotransform progesterone and studied the influence of the medium composition and temperature regime on the directionality of progesterone transformation.
MATERIALS AND METHODS
Materials. Progesterone (I) (CAS No. 57-83-0, C21H30O2) and 11α-hydroxyprogesterone (III) (CAS No. 80-75-1, C21H30O3) from Steraloids Inc. (USA), 21-hydroxyprogesterone (II) (CAS No. 64-85-7, C21H30O3) and yeast extract from Sigma-Aldrich (USA), inorganic salts from Fluka (Germany) and Amresco (USA), peptone from Bacto Difco (USA), and dimethyl sulfoxide (99.0%) from Serva (USA) were used. All other reagents and solvents (chemically pure grade and analytical grade) were purchased from Russian chemical companies.
Microorganisms and cultivation. Aspergillus niger N402 cspA genotype strain (Aspergillus niger van Tieghem ATCC 64974, anamorph (synonym FGSC A733)), a derivative of the A. niger van Tieghem ATCC 9029 strain with short conidiophores [7], was used in the study.
To obtain inoculum, the strain was grown on agar medium containing 3 g/liter malt extract, 1 g/liter peptone, and 20 g/liter agar at 28°C for 7-10 days.
Further cultivation was performed in complete medium (CM) [8] containing (g/liter): glucose, 40; MgSO4⋅7H2O, 1; KH2PO4, 0.74; peptone, 1; yeast extract, 1; L-asparagine, 0.7; and in minimal synthetic medium(MM) containing (g/liter): glucose, 20; MgSO4⋅7H2O, 0.52; KH2PO4, 1.52; NaNO3, 0.85; CuSO4⋅5H2O, 0.13; CaCl2, 0.11; KCl, 0.52; plus (in mg/liter) MnSO4⋅5H2O, 1; ZnSO4⋅7H2O, 0.8; Na2MoO4⋅4H2O, 0.8; FeSO4⋅5H2O, 0.8; plus 5 µg/liter H3BO3 in 0.1 M phosphate-citrate buffer (pH 6.6).
The microorganisms were grown at 28 or 37°C in a Brunswick Innova 44 shaker at 240-250 rpm (amplitude, 5 cm).
Biotransformation of progesterone with A. niger N402. Spore suspension was introduced into 750-ml culture flasks containing 100 ml of the cultivation medium, and the fungus was grown in a shaker at 28 or 37°C for 96 h. Progesterone solution in dimethyl sulfoxide (DMSO) was them added to the cultivation medium to the final concentration of 1 g/liter (DMSO concentration in the cultivation medium was 4%, v/v).
Progesterone transformation was performed for 192 h under the same cultivation conditions. Control samples were incubated in the absence of progesterone. All experiments were carried out in triplicate; aliquots (10 ml) of the experimental and control cell suspensions were withdrawn every 24 h (except the first aliquot that was collected 8 h after addition of the steroid). The samples of fungal cells were disintegrated with a Potter homogenizer, and the resulting homogenate was extracted three times with an equal volume of ethyl acetate. The combined extracts were washed twice with 5 ml of water each time and completely evaporated under vacuum. The residue was dissolved in 5 ml of a dichloromethane/methanol mixture (2 : 1 v/v) and analyzed for the biotransformation products by quantitative TLC on Silica gel 60 F254 TLC plates (Merck, USA).
Isolation of biotransformation products. To isolate the products of progesterone biotransformation, mycelial cells were disintegrated directly in the cultivation medium with a Potter homogenizer, and the resulting homogenate was extracted three times with an equal volume of ethyl acetate. The combined extracts were washed with water, dried with Na2SO4, and completely evaporated. The residue was dissolved in 5 ml of dichloromethane/ethyl acetate mixture (2 : 1) and subjected to chromatography on a 16 × 650-mm Silica gel 60 column (0.043-0.063 mm; Merck) using 30× amount of the sorbent to the weight of the dry residues. A mixture of dichloromethane and acetone (0-25%) was used as the eluent. The eluted fractions were analyzed for the content of biotransformation products by TLC on Silica gel 60 F254 TLC plates (Merck) in the dichloromethane/acetone solvent system (9 : 1 or 4 : 1 v/v). The biotransformation products were visualized on the plates under UV light (254 nm); the plates were then sprayed with 1% vanillin solution in 10% aqueous solution of HClO4 and developed at 100-120°C.
Identification of biotransformation products. The structure and purity of the isolated compounds were confirmed by TLC (in comparison to known standards), 1H- and 13C-NMR, and high-resolution mass spectrometry (HRMS).
The 1H- and 13C-NMR spectra were registered with a Bruker Avance-400 spectrometer (Bruker BioSpin GmbH, USA) with the working frequencies of 400 MHz for 1H-NMR and 100.6 MHz for 13C-NMR (with suppression of the carbon–proton interactions) and a Bruker Avance III spectrometer (Bruker BioSpin GmbH) with the working frequencies of 300 MHz for 1H-NMR and 75.5 MHz for 13C-NMR (with suppression of the carbon–proton interactions). The solvents used were deuterated chloroform (99.8% D; Sigma-Aldrich) or deuterated DMSO (99.9% D; Sigma-Aldrich) relative to tetramethylsilane (TMS) (NMR grade, ≥99,9%; Sigma-Aldrich) as an internal standard (δ, ppm).
High-resolution mass spectra were registered with a Bruker Daltonics micrOTOF-Q II triple quadrupole time-of-flight mass spectrometer using electrospray ionization (ESI); measurements were done for positively charged ions. The voltage on the capillary was 4500 V; range of scanned masses, m/z 50-3000; external calibration (Electrospray Calibrant Solution; Fluka, Germany); spray pressure, 0.4 bar; flow rate, 3 µl/min; nitrogen as spray gas (6 liters/min); interface temperature, 180°C. The samples were placed in the mass spectrometer spray chamber after HPLC on an Agilent 1260 chromatograph equipped with an Agilent Poroshell 120 EC-C18 column (3.0 × 50 mm; 2.7 µm) and protective cartridge using an autosampler. The samples were in 50% acetonitrile (LC-MS grade; Panreac, Spain) in water (MilliQ-purified; Merck Millipore KGaA, Germany). The samples were eluted from the column at a flow rate of 400 µl/min with a gradient of acetonitrile concentration in water in the following regime: 0-15% acetonitrile for 6 min, 15-85% acetonitrile for 1.5 min, 85-0% acetonitrile for 0.1 min, and 0% acetonitrile for 2.4 min. The retention times were: 6β,11α-dihydroxyprogesterone – 4.2 min; 11α-hydroxyprogesterone – 5.2 min; 21-hydroxyprogesterone – 5.6 min; progesterone – 6.7 min.
Melting points of the isolated compounds were determined with a Melting Point M-565 instrument (Büchi Labor Technik AG, Switzerland).
Characterization of biotransformation products. 11α-Hydroxyprogesterone: mp, 164-166°C (published mp, 164-165°C [9]).
HRMS spectrum C21H30O3 (m/z): calculated for [M+H]+ 331.2268, found 331.2264; calculated for [M+Na]+ 353.2087, found 353.2088.
1H-NMR (400 MHz, CDCl3, δ): 5.73 (s, 1H, CH-4), 4.04 (ddd (pseudo-dt), 3J11,9 = 10.3 Hz 3J11,12α 4.8 Hz 3J11,12β 4.6 Hz 1H, CH-11β), 2.66 (dt, 2J1β,1α 13.7 Hz 3J1β,2 4.4 Hz 1H, CH-1β), 2.55 (dd (pseudo-t), 3J17,16α 8.7 Hz 3J17,16β 9.1 Hz 1H, CH-17), 2.48-2.26 (m, 5H, CH2-2 and CH2-6 and CH-12β), 2.22-2.10 (m, 1H, CH-16β), 2.13 (s, 3H, CH3-21), 2.02 (td, 2J1a,1b 13.7 Hz 3J1α,2 4.5 Hz 1H, CH-1α), 1.87-1.81 (m, 1H, CH-7β), 1.78-1.64 (m, 3H, CH-8 and CH-15α and CH-16α), 1.58-1.47 (2H (t, 3J12α,12β 11.3 Hz 1H, CH-12α), 1.31 (s, 3H, CH3-19), 1.29-1.20 (m, 2H, CH-12α and CH-14), 1.14 (t 3J9,8 10.3 Hz 1H, CH-9), 1.14-1.04 (2× ddd, 3J7α,7β 13.1 Hz 3J7,6 13.0 Hz 3J7α,8 3.8 Hz 2H, CH2-7α), 0.69 (s, 3H, CH3-18).
13C-NMR (100.6 MHz CDCl3, δ): 208.91 (s, CO-20), 200.23 (s, CO-3), 170.96 (c, C-5), 124.65 (s, CH-4), 68.93 (s, CH-11), 63.21 (s, CH-17), 59.08 (s, CH-9), 55.44 (s, CH-14), 50.53 (s, CH2-12), 44.19 (s, C-13), 40.02 (s, C-10), 37.59 (s, CH2-1), 35.04 (s, CH2-2), 34.23 (s, CH2-6), 33.65 (s, CH-8), 31.65 (s, CH2-7), 31.37 (s, CH3-21), 24.31 (s, CH2-15), 23.07 (s, CH2-16), 18.40 (s, CH3-19), 14.55 (s, CH3-18).
21-Hydroxyprogesterone: mp, 141-142°C (published mp, 142-144°C [10]).
HRMS spectrum C21H30O3 (m/z): calculated for [M+H]+ 331.2268, found 331.2268.
1H-NMR (400 MHz CDCl3, δ): 5.73 (s, 1H, CH-4), 4.18 (m, 2H, CH2-21). 3,26 (wd. s., 1H, OH), 2.48-2.35 (m, 4H, CH-1β and CH-17 and CH2-2), 2.34-2.06 (m, 2H, CH2-6β and CH-12β), 2.02 (td, 2J1a,1b 13.4 Hz 3J1α,2 3.5 Hz 1H, CH-1α), 1.95-1.91 (m, 1H, CH-7β), 1.87-1.83 (m, 1H, CH- 15α), 1.79-1.29 (m, 10H, CH-8 and CH-15β and CH2-16 and CH-12α and CH-1α and CH-6α and CH-14 and CH2-11), 1.18 (s, 3H, CH3-19), 1.22-0.93 (m, 2H, CH-7α and CH-9), 0.68 (s, 3H, CH3-18).
13C-NMR (100.6 MHz CDCl3, δ): 210.24 (s, CO-20), 199.55 (s, CO-3), 170.86 (s, C-5), 124.04 (s, CH-4), 69.47 (s, CH-21), 59.08 (s, CH-17), 56.12 (s, CH-14), 53.61 (s, CH-9), 44.72 (s, C-13), 38.62 (s, C-10), 38.42 (s, CH2-12), 35.75 (s, CH2-1), 33.59 (s, CH-8), 33.91 (s, CH2-2), 32.78 (s, CH2-6), 31.92 (s, CH2-7), 24.52 (s, CH2-15), 22.99 (s, CH2-16), 20.90 (s, CH2-11), 17.42 (s, CH3-19), 13.52 (s, CH3-18).
6β,11α-Dihydroxyprogesterone: mp, 242-244°C (published mp, 244-246°C [11]).
HRMS spectrum C21H30O4 (m/z): calculated for [M+H]+ 347.2217, found 347.2212; calculated for [M+Na]+ 364.2482, found 364.2482.
1H-NMR (300 MHz DMSO-d6): 5.65 (s, 1H CH-4), 5.08 (wd. s, 1H, OH), 4.35 (wd. s, 1H, OH), 4.14 (wd. s, 1H, CH-6), 3.86 (ddd (pseudo-dt), 1H, 3J11,9 10.4 Hz 3J11,12α 4.6 Hz 3J11,12β 4.4 Hz CH-11), 2.75 (ddd (pseudo-td of protons X in the ABXY system), 1H, 2J1a,1b 13.7 Hz 3J1a,2a 3.6 Hz 3J1a,2b 3.3 Hz CH-1β), 2.61 (dd (pseudo-t), 1H, 3J17,16a 8.5 Hz 3J17,16b 8.8 Hz CH-17), 2.43 (ddd, 1H, 2J2a,2b 14.7 Hz 3J2a,1a 4.5 Hz 3J2a,1b 3.6 Hz CH-2β), 2.18 (m, 2H, CH-2α and CH-12β), 2.08 (wd. s, 4H, CH3-21 and CH-16β), 1.92-1.86 (m, 1H, CH-8), 1.86-1.84 (m, 2H, CH-1α and CH-7β), 1.67-1.57 (m, 2H, CH-16α and CH-15α), 1.47 (dd (pseudo-t), 1H, 3J12α,11 10.4 Hz 3J12α,12β 11.6 Hz CH-12α), 1.39 (s, 3H, CH3-19), 1.30-1.08 (m, 3H, CH-7α and CH-15β and CH-14), 1.00 (m (pseudo-t), 1H, CH-9), 0.60 (s, 3H, CH3-18).
13C-NMR (300 MHz DMSO-d6): 208.14 (s, CO-20), 199.63 (s, CO-3), 169.56 (s, C-5), 125.51 (s, CH-4), 71.22 (s, CHOH-6), 67.12 (s, CHOH-11), 62.37 (s, CH-17), 58.20 (s, CH-9), 54.72 (s, CH-14), 49.33 (s, CH2-12), 43.61 (s, C-13), 38.87 (s, C-10), 38.55 (s, CH2-1), 37.98 (s, CH2-7), 34.05 (s, CH2-2), 30.92 (s, CH3-21), 27.94 (s, CH-8), 23.83 (s, CH2-15), 22.25 (s, CH2-16), 19.57 (s, CH3-19), 14.16 (s, CH3-18).
RESULTS AND DISCUSSION
In this work we investigated the ability of A. niger strain N402 to transform progesterone. Progesterone is commonly used as a model compound in studies of steroid hydroxylation by mycelial fungi. We also assessed the effects of medium composition and cultivation temperature on the hydroxylation activity of A. niger and directionality of progesterone hydroxylation.
The effect of the cultivation medium composition on the targeted hydroxylation of progesterone was studied at two different temperatures (28 and 37°C) in two different media: complete medium (CM) that has been described by El-Refai et al. [12] as the most favorable for progesterone conversion into its 11α-hydroxyderivative by Aspergillus nidulans, and minimal medium (MM) that contained 0.5 mM CuSO4. Cultivation of A. niger and the process of progesterone transformation were studied in the same temperature regime.
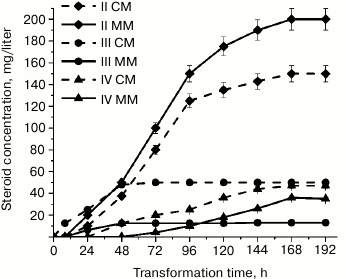
We found that at 28°C, A. niger N402 exhibited hydroxylating activity and introduced a hydroxyl group into the progesterone molecule at positions C21 and C11(α) in both CM and MM. The by-products of this biotransformation were 6β,11α-dihydroxyprogesterone and four minor hydroxylation products that were synthesized in amounts insufficient for their identification. The formation of 21-hydroxyprogesterone (II), 11α-hydroxyprogesterone (III), and 6β,11α-dihydroxyprogesterone (IV) by A. niger N402 was confirmed by 1H-NMR and 13C-NMR spectroscopies and chromatography coupled with high-resolution mass spectrometry. Figure 1 shows the kinetics of accumulation of progesterone biotransformation products in CM and MM at 28°C.
Fig. 1. Accumulation of progesterone biotransformation products in MM (solid line) and CM (dashed line) for 192 h at 28°C: rhombi, 21-hydroxyprogesterone (II); circles, 11α-hydroxyprogesterone (III); triangles, 6β,11α-dihydroxyprogesterone (IV).
After 8 h of cultivation, no biotransformation products were identified in MM, whereas CM contained only small amounts of III (Fig. 1). After 24 h of cultivation, the concentration of III in CM increased two times; the medium also contained trace quantities of II. In contrast, in MM the content of II was higher than the content of III. Both media lacked the secondary biotransformation product IV; however, it appeared in CM when A. niger N402 was cultivated for more than 24 h. After 72 h of cultivation, the content of IV in CM increased almost two times; only trace amounts of IV were found in MM. However, accumulation of II in this medium was more intensive than in CM. El-Refai et al. [12] observed formation of IV during progesterone biotransformation by A. nidulans under similar conditions and concluded that at pH close to neutral, 6β-hydroxylation is a secondary process and 6β,11α-dihydroxyprogesterone is formed from the previously synthesized 11α-hydroxyprogesterone.
Therefore, in both tested media progesterone biotransformation by A. niger N402 at 28°C was shifted toward 21-hydroxylation. Compound IV appeared in CM in trace amounts only after 24 h of biotransformation, in MM – after 48 h, which may have resulted from the inhibition of 6β-monooxygenase activity in MM. It should be noted that the maximum accumulation of III was observed only after 48 h of transformation irrespectively of the medium composition and then stayed at the same level. On the contrary, accumulation of II continued up to 168 h of incubation in both media.
Apparently, the reactions of 11α- and 21-hydroxylation of progesterone are catalyzed by different enzymes. In some Aspergillus fungi, these processes are catalyzed by cytochrome P450-dependent monooxygenases that belong to a multienzymatic oxidative complex, namely progesterone 11α-monooxygenase (EC 1.14.99.14) and steroid 21-monooxygenase (EC 1.14.99.10) [13, 14].
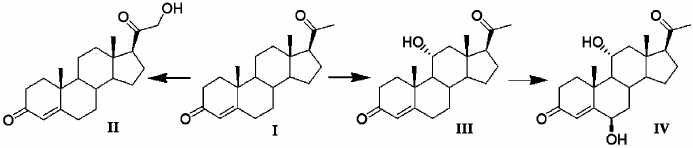
Figure 2 shows the reactions of progesterone transformation by A. niger N402.
Fig. 2. Transformation of progesterone by A. niger N402: I, progesterone; II, 21-hydroxyprogesterone; III, 11α-hydroxyprogesterone; IV, 6β,11α-dihydroxyprogesterone.
Earlier research showed that the presence of copper ions in the growth medium inhibits steroid C21-monooxygenase (EC 1.14.99.10) [13]. However, our experiments demonstrated that 0.5 mM CuSO4 in the growth MM did not suppress steroid C21-monooxygenase activity of A. niger N402. On the contrary, the content of II in the copper-containing MM was considerably higher than in CM containing no Cu2+ probably due to the activation of 21-monooxygenase in the presence of Cu2+. The activity of 11α-monooxygenase was suppressed, thereby indicating different sensitivity of these two enzymes to metal ions. 6β-Hydroxylase was also inhibited in MM, which correlates with the results of El-Kady [3] who related this inhibition to the presence of Co2+ and Cd2+ in the medium.
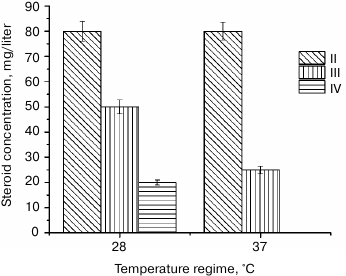
Another factor that determines the yield of biotransformation products is the cultivation conditions (e.g. temperature) [12]. Here we studied the effect of temperature increase to 37°C on the quantitative and qualitative content of progesterone biotransformation products after 72 h of transformation (Fig. 3).
Fig. 3. Accumulation of progesterone biotransformation products in CM after incubation for 72 h at 28 and 37°C.
We found that A. niger N402 did not transform progesterone in MM at 37°C, most probably because of the absence of steroid monooxygenase activity under these conditions. Increasing the temperature from 28 to 37°C did not affect accumulation of II in CM (Fig. 3), but the content of III decreased twice, and IV was absent. Therefore, we concluded that the enzymes responsible for hydroxylation of progesterone molecules at different position differ in their sensitivity to temperature. The temperature increase did not affect the activity of 21-monooxygenase, but it significantly inhibited 11α-hydroxylase, and completely inactivated 6β-hydroxylase. Our results correlate well with the data of Kim et al. [15], who studied the effect of the temperature regime (25 to 50°C) on the content of progesterone transformation products by Aspergillus phoenicis and showed that accumulation of 11α-hydroxyprogesterone (III) and 6β,11α-dihydroxyprogesterone (IV) was maximal at 28°C after 20 h. When A. phoenicis was incubated for the same time at 40°C, the content of 11α-dihydroxyprogesterone (III) decreased three times, while a 6β,11α-dihydroxyprogesterone (IV) was completely absent.
Therefore, we demonstrated that A. niger N402 exhibits steroid-hydroxylating activity and modifies the progesterone molecule at two positions (C11(α) and C21) with subsequent 6β-hydroxylation of 11α-hydroxyprogesterone. In both tested media, the main product of hydroxylation was 21-hydroxyprogesterone. When biotransformation was carried out at 28°C for 168 h, the amount of 21-hydroxyprogesterone in MM was 33% higher than in CM, and formation of 11α-hydroxyprogesterone was significantly inhibited. Increasing the temperature to 37°C did not affect 21-hydroxylating activity in CM but suppressed 11α-hydroxylating activity and completely inhibited 6β-hydroxylating activity, which indicates different temperature sensitivity of the corresponding enzymes. We believe the results of this study to be of a great practical significance because they would allow control of the hydroxylation process and its selectivity to preferentially synthesize required hydroxy derivative to facilitate purification of the target product.
Acknowledgments
The authors thank R. A. Novikov, Engelhardt Institute of Molecular Biology, for registering NMR spectra.
This work was supported by the Russian Foundation for Basic Research (project No. 17-04-00536-a; Identification of biotransformation products by chromato-mass-spectrometry).
REFERENCES
1.Mashkovsky, M. D. (2005) Drugs: Handbook for
Physicians [in Russian], Novaya Volna, Moscow, pp. 579-580.
2.Numazawa, M., and Nagaoka, M. (1983) A facile
synthesis of deoxycorticosterone using the controlled alkaline
hydrolysis of 21-bromo-20-ketopregnenes, J. Chem.
Soc. Chem. Commun., 3, 127-128.
3.El-Kady, I. A. (1982) 6b-Hydroxylation of steroids
by extracts of Aspergillus niger, J. Gen.
Microbiol., 128, 2511-2514.
4.Fouad, W. A., Abbas, I. H., Elwan, K. M., Swellum,
M. A., and El-Dougdoug, Kh. A. (2009) Biotransformation of progesterone
by microbial steroids, J. Appl. Sci. Res.,
35, 137-143.
5.Metwali, M. R. (2002) Microbiological
transformation of progesterone to 21-hydroxyprogesterone by
Aspergillus niger (isolate No. 11/3), Bulletin of the Faculty
of Science, Assiut University, D Botany,
31, 313-318.
6.Koolman, J., and Rohm, K.-H. (2009) Taschenatlas
der Biochemie, Thieme.
7.Bos, C. J., Debets, A. J. M., Swart, K., Huybers,
A., Kobus, G., and Slakhorst, S. M. (1988) Genetic analysis and the
construction of master strains for assignment of genes to six linkage
groups in Aspergillus niger, Curr. Genet.,
14, 437-443.
8.Capek, A., Tadra, M., and Tuma, J. (1964) Microbial
transformations of steroids XXIV. Separation of androstane
17-hydroxy-epimers, Folia Microbiol., 9,
380-382.
9.Yildirim, K., and Kuru, A. (2015) Biotransformation
of some steroids by Aspergillus candidus, J. Chem.
Res., 39, 546-549.
10.Hosseinabadi, T., Vahidi, H., Nickavar, B., and
Kobarfard, F. (2015) Biotransformation of progesterone by whole cells
of filamentous fungi Aspergillus brasiliensis, Iran J.
Pharm. Res., 14, 919-924.
11.Habibi, Z., Yousefi, M., Ghanian, Sh., Mohammadi,
M., and Ghasemi, S. (2012) Biotransformation of progesterone by
Absidia griseolla var. igachii and Rhizomucor
pusillus, Steroids, 77, 1446-1449.
12.El-Refai, A. H., and Ghanem, K. M. (1987) Some
physiological relations of progesterone conversion by Aspergillus
nidulans, Egyptian J. Microbiol., 22,
327-338.
13.Schomburg, D., and Schomburg, I. (2006)
Springer Handbook of Enzymes. Vol. 27. Class 1 –
Oxidoreductases XII, 2nd Edn., Springer Verlag, Berlin, p. 302.
14.Samanta, T. B., and Ghosh, D. K. (1987)
Characterization of progesterone 11 alpha-hydroxylase of Aspergillus
ochraceus TS: a cytochrome P-450 linked monooxygenase, J. Steroid
Biochem., 28, 327-332.
15.Kim, M.-N., Lee, Y.-J., and Lee, H.-H. (1985)
Steroid modification with Aspergillus phoenicis. Effects of
reaction temperature and sonication, Kor. J.
Mycol., 13, 83-87.