Myoglobin: Oxygen Depot or Oxygen Transporter to Mitochondria? A Novel Mechanism of Myoglobin Deoxygenation in Cells (review)
G. B. Postnikova* and E. A. Shekhovtsova
Institute of Cell Biophysics, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; E-mail: gb_post@icb.psn.ru* To whom correspondence should be addressed.
Received April 24, 2017; Revision received September 15, 2017
In this review, we shortly summarize the data of our studies (and also corresponding studies of other authors) on the new mechanism of myoglobin (Mb) deoxygenation in a cell, according to which Mb acts as an oxygen transporter, and its affinity for the ligand, like in other transporting proteins, is regulated by the interaction with the target, in our case, mitochondria (Mch). We firstly found that contrary to previously formulated and commonly accepted concepts, oxymyoglobin (MbO2) deoxygenation occurs only via interaction of the protein with respiring mitochondria (low pO2 values are necessary but not sufficient for this process to proceed). Detailed studies of the mechanism of Mb–Mch interaction by various physicochemical methods using natural and artificial bilayer phospholipid membranes showed that: (i) the rate of MbO2 deoxygenation in the presence of respiring Mch fully coincides with the rate of O2 uptake by mitochondria from a solution irrespectively of their state (native coupled, freshly frozen, or FCCP-uncoupled), i.e. it is determined by the respiratory activity of Mch; (ii) Mb nonspecifically binds to membrane phospholipids of the outer mitochondrial membrane, while any Mb-specific protein or phospholipid sites on it are lacking; (iii) oxygen uptake by Mch from a solution and the uptake of Mb-bound oxygen are two different processes, as their rates are differently affected by proteins (e.g. lysozyme) that compete with MbO2 for binding to the mitochondrial membrane; (iv) electrostatic forces significantly contribute to the Mb–membrane interactions; the dependence of these interactions on ionic strength is provided by the local electrostatic interactions between anionic groups of phospholipids (the heads) and invariant Lys and Arg residues near the Mb heme pocket; (v) interactions of Mb with phospholipid membranes promote conformational changes in the protein, primarily in its heme pocket, without significant alterations in the protein secondary and tertiary structures; and (vi) Mb–membrane interactions lead to decrease in the affinity of myoglobin for O2, which could be monitored by the increase in the MbO2 autooxidation rate under aerobic conditions and under anaerobic ones, by the shift in the MbO2/Mb(2) equilibrium towards the ligand-free protein. The decrease in the affinity of Mb for the ligand should facilitate O2 dissociation from MbO2 at physiological pO2 values in cells.
KEY WORDS: myoglobin, mitochondria, spatial structure, deoxygenation mechanismDOI: 10.1134/S0006297918020098
Abbreviations: apoMb, apomyoglobin; BLM, bilayer lipid membranes; BSA, bovine serum albumin; DMb, Mb diffusion coefficient; DPPG, 1,2-dipalmitoylphosphatidylglycerol; FCCP, carbonylcyanide-4-(trifluoromethoxy)phenylhydrazone; GuHCl, guanidine chloride; Kdis, complex–ligand equilibrium dissociation constant; KM, Mb binding constant to mitochondria; kox, MbO2 autooxidation rate constant; M540, merocyanine 540; Mb, myoglobin; Mb(2), deoxymyoglobin; MbO2, oxymyoglobin; Mch, mitochondria; metMb, metmyoglobin; pO2, oxygen partial pressure; p50, pO2 value at which Mb(2) is half-oxygenated (myoglobin affinity for oxygen); POPG, 1-palmitoyl-2-oleylglycero-3-phosphoglycerol; ROS, reactive oxygen species; SMbO2, extent of myoglobin saturation with oxygen.
Myoglobin (Mb) is a monomeric cytoplasmic hemoprotein (17,800 Da)
that was discovered in the beginning of the 20th century [1, 2]. It is synthesized in
cardiomyocytes and skeletal muscle cells. Since Mb could be easily
purified in large amounts, it was the first protein whose 3D structure
was solved with atomic resolution [3]. Structural
and functional properties of Mb have been studied in detail and
described in various textbooks, reviews, and monographs [4-6]. However, Mb is still
extensively studied because it is often used as a model protein for
approbation of new experimental and theoretical approaches in
biochemistry and molecular biology.
Concentration of Mb is especially high in muscles of mountain animals and marine mammals that spend considerable amounts of time underwater in search of food. This led to the suggestion that Mb plays a role of the “oxygen depot” for the mitochondrial cytochrome c oxidase that serves to compensate O2 deficit during muscle contractions, when pO2 in the cells falls below the critical level [2]. Because Mb concentration in muscles of terrestrial animals is low (according to calculations, the amount of stored O2 is sufficient only for 6 s, i.e. for one contraction cycle), it was suggested that Mb facilitates oxygen diffusion via reversible binding of O2 molecule followed by translational diffusion of MbO2 in the cell [7-11]. Since the origin of this hypothesis, physiological significance of “the facilitated diffusion” has been widely debated. Some authors in their experimental and theoretical studies corroborate large contribution of this mechanism to the cell supply with O2, while others completely reject it [12-16]. Both mechanisms, “the oxygen depot” and “the facilitated diffusion”, are formulated within the framework of homogenous thermodynamics and principles of reaction kinetics in solutions and do not assume any interaction between Mb and cell structures or metabolites, so that the myoglobin affinity for O2 in all calculations is considered constant [11, 15, 17].
Why have MYOGLOBIN and the mechanism of its functioning become hot topics again?
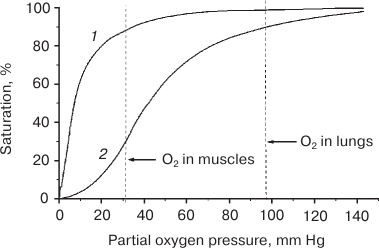
Since the studies of Hill [1] and Millikan [2], it has been commonly accepted that similarly to blood hemoglobin, muscle myoglobin functions according to its oxygenation curve in solution (Fig. 1, curve 1). Because of the high affinity of deoxyhemoglobin Mb(2) for O2, the equilibrium is strongly shifted toward MbO2:
where k1 and k–1 are constants for O2 binding and dissociation, respectively.
Fig. 1. Oxygenation curves of myoglobin (1) and hemoglobin (2) [5].
The value of p50, which is a measure of the affinity of Mb for the O2 ligand, varies for different animal Mbs and is 0.5-1.0 and 2.0-2.75 mm Hg at 20-25 and 37°C, respectively [4, 5]. Therefore, efficient deoxygenation of MbO2 in muscle cell requires very low pO2 values (< 3 mm Hg). But the pO2 values found now in contracting muscles are much higher and can reach, according to different sources, 11.4 to 25 mm Hg, so that Mb saturation with O2 (SMbO2) in a hardly working heart does not decrease below 85% and comprises 92% of total protein pool [13, 14, 18]. It should be noted that no cell regulatory molecules have been so far found that decrease Mb affinity for the ligand and, hence, are able to shift the oxygenation curve toward higher pO2 values. That differs monomeric Mb from tetrameric hemoglobin, whose affinity for O2 is modulated by the ligand itself and allosteric regulators, such as protons, CO2, and diphosphoglycerate [19] to provide the required rate of O2 delivery by the blood.
The facilitated diffusion mechanism would be also efficient only at very low pO2 and SMbO2 values, i.e. under conditions that do not exist in cells. Moreover, any noticeable contribution of “the facilitated diffusion” to supplying cells with O2 during intense muscle work suggests the existence of a steep MbO2 gradient in a cell, which is possible only at low SMbO2 values (that are not observed) and high Mb diffusion coefficients. However, DMb values experimentally observed in skeletal muscles are 5-10 times lower than the values originally used for calculating the contribution of “the facilitated diffusion” to oxygen supply in skeletal muscles. Thus, this contribution equals only 1.5-4% at pO2 < 13 mm Hg and becomes negligibly small at pO2 > 13 mm Hg [20, 21].
Therefore, efficient functioning of myoglobin according to the accepted “the oxygen depot” and “the facilitated diffusion” mechanisms is very problematic. About 20 years ago, the concept of Mb as O2 supplier to Mch received a devastating blow after a sensational communication that transgenic mice with knocked-out Mb gene do not differ from their wild-type counterparts in standard physiological and histological tests [22]. Both Mb-deficient and control mice develop and reproduce normally and tolerate increased muscle loads without changes in the blood vessels or the mitochondria content in myocytes. A question has arisen: if Mb is necessary and, if yes, what for [23]? It is possible that it serves not for an uninterrupted supply of O2 to mitochondria for ATP production, but is involved in other cellular processes, such as regulation of NO level, antioxidant protection of tissues, or even transport of fatty acids [24]. Indeed, it was found that mutant myo–/– mice exhibited NO-dependent weakening of cardiac functions and were more sensitive to ROS-induced oxidative stress after ischemia/reperfusion of myocardium compared to control animals [25-27].
It should be noted that in recent years, new properties of Mb have been described that might be of physiological importance. Thus, it was found that Mb has NO deoxygenase activity, oxidizing NO into nitrite and forming metMb. This suggests that in a working muscle, NO might bind to the MbO2 heme and prevent NO-induced inhibition of mitochondrial respiration, thereby affecting oxidative phosphorylation in a cell [28]. At the same time, Mb(2) on the contrary can act as a potential nitrite reductase, i.e. promote formation of NO and metMb, and shift the reaction from NO utilization to its generation. Although nitrite by itself does not affect the respiration of isolated Mch, it inhibits the respiratory chain in cardiomyocytes in the presence of Mb [29]. The involvement of Mb in both NO utilization and formation is regulated by intracellular pO2, which is close to p50 of Mb [30].
It was also found that ferrous and ferric myoglobins interact in vitro with the superoxide anion O2–· and hydrogen peroxide H2O2 (terminal product of superoxide anion disproportionation) [31, 32]. Since ROS are the most important factor in tissue damage during oxidative stress, the in vivo role of Mb as a peroxidase might be ROS elimination and antioxidant protection of cells [27, 33]. Finally, various Mb ligand forms are well known to bind fatty acids, which suggests Mb involvement in fatty acid metabolism [34-37].
By now, the idea that the main function of Mb is supplying myocytes with oxygen has been rehabilitated, because it was shown that in Mb-deficient mice, hypoxia induces development in ontogenesis of numerous compensatory mechanisms that increase energy production [38-41]. Apparently, these mechanisms provide normal functioning of muscle cells when less-energy consuming Mb-dependent transport of O2 to cells is impossible. However, it cannot be excluded that recently discovered new globins, such as monomeric neuroglobins (Ngbs) that are produced in the brain in small amounts and dimeric cytoglobins (Cgbs) that are present in virtually all nonmuscle tissues, act mostly as protectors of cells against oxidative stress and not as oxygen carriers [42-44]. Unlike muscle myoglobin and blood hemoglobin, Ngbs and Cgbs contain six-coordinated heme, in which the sixth ligand of the Fe atom is the distal HisE7. Despite the capacity for oxygen binding, Ngbs and Cgbs are present in very small amounts (especially Ngbs), so that they most probably do not play any significant role in oxygen consumption by the cells. Interestingly, low concentrations of Mb start to express in nonmuscle cells in response to hypoxia [45].
The problem of how Mb functions has been resolved as well. It was shown that oxygen dissociation from MbO2 with the formation of the ligand-free Mb(2) at physiological pO2 values proceeds only via direct contact of Mb with respiring mitochondria, and the mechanism of the Mb–Mch interaction was studied in detail [46-51]. According to this mechanism, Mb is the oxygen transporter and, similarly to other transporting proteins, its affinity for O2 is regulated by the interaction with target structures, i.e. mitochondria. This new mechanism is free from the contradictions faced by “the oxygen depot” and “the facilitated diffusion” theories and allows correct estimation of the contribution of Mb to the cell supply with oxygen. Knowledge of the true mechanism of Mb functioning is important not only from the theoretical point of view but might have practical applications. For example, as cell culturing requires optimal conditions for supply cells with oxygen, it would be useful to have a correct model for estimating the necessity for the introduction of Mb-expressing genes and cells into the culture, which is expensive and makes the whole process very costly [17].
New mechanism for myoglobin deoxygenation in cells
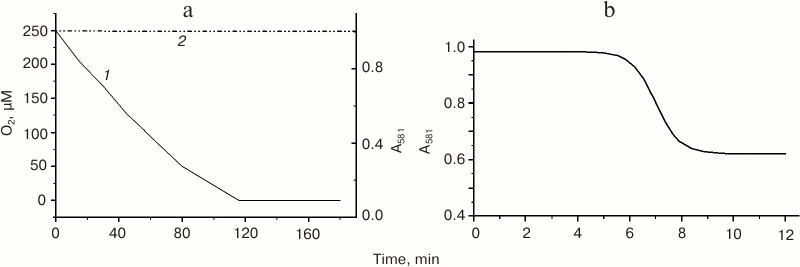
Oxygen consumption from solution by mitochondria and MbO2 deoxygenation in the presence of mitochondria. We were the first to demonstrate [46] that oxygen dissociation from MbO2 al low physiological pO2 values with the formation of ligand-free Mb(2) takes place only upon direct contact of MbO2 with respiring mitochondria. If the mitochondria are separated from the MbO2 solution with a semipermeable membrane (i.e. placed in a sealed dialysis bag in a closed polarographic cell), no MbO2 deoxygenation happens even at O2 concentrations close to zero (Fig. 2a). Spectrophotometrically registered rate (V2) of MbO2 deoxygenation in the presence of mitochondria (Fig. 2b) completely coincides with the polarographically registered rate of oxygen consumption by the mitochondria (V1) in the presence of MbO2 for all the mitochondria states studied: native coupled mitochondria with low rate of succinate respiration (V0) and preserved oxidative phosphorylation; FCCP-treated uncoupled mitochondria with the maximal V0; and frozen native mitochondria with an intermediate respiration rate. This implies that the rate of MbO2 deoxygenation (V2) in the presence of mitochondria is entirely determined by the respiratory activity of mitochondria (Table 1). This rate is constant during all the transition (Fig. 2b), does not depend on MbO2 concentration (zero order in Mb), and is proportional to the concentration of mitochondria.
Fig. 2. a) Kinetics of O2 consumption by frozen rat liver mitochondria in a polarographic cell (curve 1, solid line) and changes in the MbO2 absorbance spectrum at 581 nm (curve 2, dashed line) [47]. Experimental conditions: 0.9 ml of Mch (30 mg mitochondrial protein/ml) in a sealed dialysis bag (impenetrable for proteins) were placed in a closed polarographic cell containing 12.5 ml of 0.11 mM MbO2 solution. Incubation medium: 150 mM sucrose, 100 mM KCl, 0.5 mM EGTA, 5 mM KH2PO4, 15 mM succinate, and 10 mM MOPS, pH 7.4. b) Kinetics of O2 dissociation from MbO2 in a suspension of frozen rat liver Mch [46, 47]. Absorbance was measured at 581 nm with a Specord UV VIS spectrophotometer using the cell for turbid samples to decrease the effect of light scattering. MbO2 concentration was 0.11 mM; mitochondrial protein concentration was 1 mg/ml. The incubation medium was the same as in Fig. 2a.
Table 1. Rates of O2 consumption
(V1) by rat liver mitochondria in the presence of
MbO2 (measured polarographically) and of MbO2
deoxygenation (V2) in the presence of mitochondria
(measured spectrophotometrically) [47]
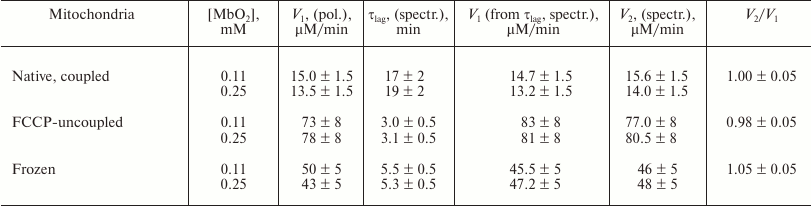
Note: Incubation medium – 150 mM sucrose, 100 mM KCl, 0.5 mM EGTA, 5 mM KH2PO4, 10 mM MOPS (pH 7.4), 15 mM succinate, and 1 mg/ml mitochondrial protein. Native coupled mitochondria with the succinate respiration rate, Vmin; native FCCP-uncoupled mitochondria (FCCP concentration, 0.5 µM) with the maximal succinate respiration rate, Vmax; respiratory control, Vmax/Vmin = 4.5-5.0; flash-frozen at –18°C and thawed native mitochondria [46, 47].
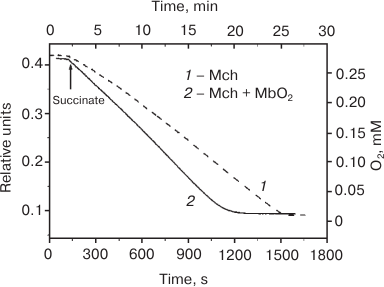
We also discovered [46] that the rate of O2 consumption by the mitochondria in a polarographic cell (V0) increases in the presence of MbO2, but not metMb; i.e. V1 > V0 (uncoupling effect) (Fig. 3). The concentration of MbO2 in our experiments (0.25 mM) was similar to the MbO2 concentration in the cytoplasm of myocytes from terrestrial mammals (0.3-0.5 mM); and the amount of oxygen bound to MbO2 was comparable to its concentration in the saturated water solution. Because the polarographic electrode did not register any increase in the O2 concentration resulting from oxygen dissociation from MbO2 (Fig. 3), we concluded this oxygen directly entered the mitochondria on contact of the MbO2 with the mitochondrial membrane.
Fig. 3. Rate of O2 consumption by frozen rat liver mitochondria (Mch) (mitochondrial protein concentration, 0.35 mg/ml) in a polarographic cell in the absence (curve 1) and presence (curve 2) of MbO2 in the solution [46]. MbO2 concentration is 0.25 mM; standard incubation medium (see Fig. 2a).
The uncoupling effect of MbO2 in native coupled mitochondria is slight (10-15% V0) but reproducible in solutions with low or high ionic strength. For the preparations of frozen mitochondria, the effect of MbO2 in the presence of 100 mM KCl was twice as high (25-30% V0) and reached its maximum (40-50% V0) in the salt-free medium. In the absence of MbO2, no effect of the ionic strength on V0 was observed in all the mitochondrial preparations tested, thereby proving that the uncoupling action of MbO2 is determined by its interaction with the mitochondria. However, it remains obscure how MbO2 influences the respiratory chain that is localized in the inner mitochondrial membrane, while the outer membrane is impenetrable for proteins. We cannot exclude that the uncoupling effect of MbO2 is required for heat generation in hibernating animals during winter hibernation (Mb does not affect ATP synthesis). It was found that the content of Mb in the muscles of the hibernating Yakut ground squirrel is 2.5-3.0 times higher in winter and 1.8-2.0 times higher in autumn than during the summer period [52-54].
The results of later studies of deoxygenation of six monomeric oxyglobins – horse Mb, Busycon and Lucina hemoglobins I and II, soybean leghemoglobin c1 and Gasterophilus hemoglobin – in the presence of pig heart Mch using glutamate and malate as respiratory substrates [55], in our opinion, prove the interaction between these globins and Mch, although the authors of that study reject this idea. But according to the data presented in [55], all studied oxyglobins demonstrated similar deoxygenation rates that correlated with the respiration rate of Mch and did not depend on kinetic and equilibrium parameters for the reaction of these proteins with O2 in solution differed by tens or even a hundred times. The observations made in [55] also corroborate the uncoupling effect of MbO2 on the rate of oxygen consumption by mitochondria found by us earlier.
Myoglobin is formed at the mitochondrial membrane surface from the apoprotein and the heme. Immunohistochemical analysis and electron microscopy confirmed localization of Mb in the vicinity of mitochondria. The presence of Mb in the mitochondrial fraction was also demonstrated by Western blotting [56]. The interaction between Mb and Mch was indicated by the fact that in the presence of Mch and succinate as a respiratory substrate, metMb could be reduced to Mb(2) [57]. In the absence of succinate or upon inhibition of the respiratory chain with antimycin A, MbO2 could be in turn oxidized into metMb [58]. In both cases, the redox reaction is believed to take place in the intermembrane space or at the surface of the outer mitochondrial membrane with the involvement of mitochondrial cytochrome c [57, 58].
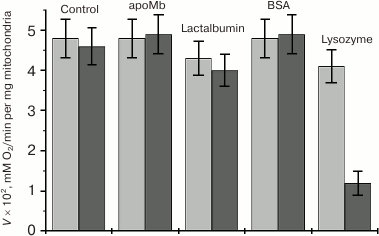
Competitive effect of other proteins on the rate of MbO2 deoxygenation in a mitochondria suspension. To identify Mb-interacting proteins, channels, or phospholipid fragments in the outer mitochondrial membrane, the competitive effect of apomyoglobin (apoMb) on the rate of MbO2 deoxygenation in the presence of mitochondria (V2) was studied. ApoMb is structurally homologous to the holoprotein but lacks the heme [47]. The effect of Coulomb electrostatics on the interaction between Mb and negatively charged outer mitochondrial membrane was estimated by studying the competitive effect on V2 of positively and negatively charged proteins, such as lysozyme (14.2 kDa), lactalbumin (14.02 kDa), and bovine serum albumin (69.0 kDa). Control experiments showed that none of these proteins at a concentration of 0.25 mM influenced the respiratory activity (V0) of native and frozen mitochondria, i.e. unlike MbO2, did not uncouple mitochondria.
Under standard conditions, apoMb does not inhibit oxygen dissociation from MbO2, which indicates that the outer mitochondrial membrane does not contain Mb-specific proteins or channels. It is also highly improbable that this membrane has any Mb-specific phospholipid fragments, since more hydrophobic apoMb should have bound to these fragments better than the holo protein. Negatively charged lactalbumin (pI 4.4) and BSA (pI 4.7) also did not change V2 under standard conditions, i.e. did not compete with MbO2 for the binding with the mitochondrial membrane.
Unlike other tested proteins, positively charged lysozyme (pI 11) significantly (~70%) inhibited MbO2 deoxygenation already at equimolar concentration (Fig. 4). This pronounced inhibitory effect suggests that lysozyme strongly competes with MbO2 for the binding to the mitochondrial membrane and that electrostatic interactions play an important role in this process. The fact that lysozyme did not change V0 favors the idea that the O2 uptake by mitochondria from solution and consumption of oxygen from MbO2 are two different processes whose rates are differently influenced by the studied proteins.
Fig. 4. Effect of proteins on the rate of O2 consumption (V1) by frozen mitochondria in MbO2 solution measured polarographically (gray bars) and on the rate of MbO2 deoxygenation in the presence of mitochondria (V2) measured spectrophotometrically (black bars) [47]. Concentration of all tested proteins was 0.25 mM; MbO2 concentration in the solution was 0.11 mM; incubation medium and experimental conditions were as in Fig. 2a.
What does myoglobin interact with at the mitochondrial membrane: proteins or phospholipids? The role of electrostatic forces in Mb interactions with mitochondria. Quenching of mitochondrial fluorescence by myoglobin. Since the heme group is a strong quencher of fluorescence of various donor molecules, we studied quenching of the intrinsic flavin and tryptophan fluorescence of Mch and the fluorescence of mitochondria-incorporated lipid probe merocyanine 540 (M540) [48]. Physiologically active MbO2 and oxidized metMb incapable of oxygen binding were used as quenchers. Because the heme group is not located at the Mb surface but imbedded into the protein, quenching by the dynamic mechanism was impossible, and energy transfer to the heme could happen only within the Mb–mitochondria complex, the life time of which exceeded the lifetime of the donor fluorescence (static quenching).
Neither MbO2 nor metMb quenched intrinsic tryptophan and flavin fluorescence of the mitochondrial suspension at pH 6-8. The critical distance for resonance energy transfer (the Förster radius) in the flavin–heme and tryptophan–heme pairs was ~5.0 and ~3.7 nm, respectively [59]. Since flavins are components of proteins of the electron transport chain in the inner mitochondrial membrane, and MbO2 and metMb cannot directly interact with the respiratory chain proteins because of the impermeability of the outer mitochondrial membrane for proteins, the absence of fluorescence quenching was trivial and suggested physical integrity of the studied mitochondria.
The absence of intrinsic tryptophan fluorescence quenching indicated that mitochondrial proteins, which occupy 50% of the outer membrane surface, did not form any stable complexes with MbO2 and metMb. This correlates well with the fact that databases of stable protein–protein complexes do not contain any data on such complexes for Mb [60]. In Mb itself, fluorescence of both conserved tryptophan residues, Trp7(A5) and Trp14(A12), located 2.15 and 1.5 nm, respectively, from the heme, is almost completely quenched by the heme complex (the quantum yield of tryptophan fluorescence in the holoprotein was only 1-5% of the quantum yield of tryptophan fluorescence in water) [61].
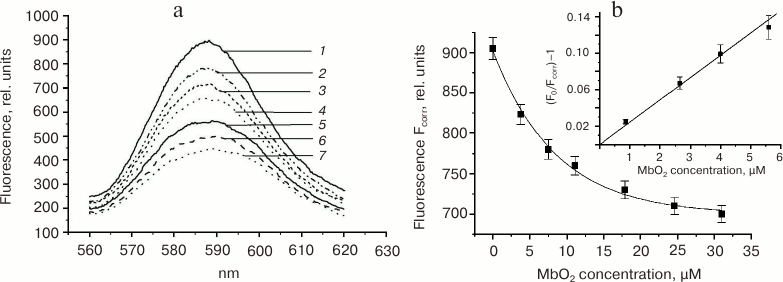
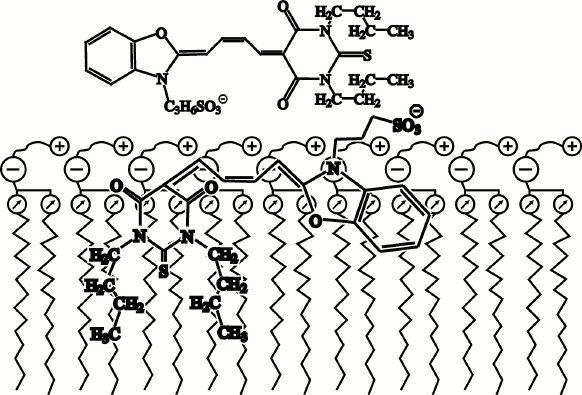
On the contrary, fluorescence of M540 lipid probe was efficiently quenched by both MbO2 and metMb (Fig. 5, a and b), which suggests the myoglobin binding with the phospholipid fragments of the outer mitochondrial membrane. The M540 probe, which contains a negatively charged sulfonic group, localizes in the intermediate region of the phospholipid bilayer polar heads (Fig. 6) [59, 62, 63]. The lifetime of the quenching complex should be >2 ns [63]. Low extent of fluorescence quenching (30 ± 10%) could be explained if not all bound probes formed complexes with the quenchers because of the heterogenous character of binding sites and/or improper mutual orientation of the donor and the acceptor (Table 2).
Fig. 5. a) Quenching of fluorescence of the mitochondria-bound M540 probe with MbO2 (succinate-free medium: 250 mM sucrose, 0.5 mM EGTA, 5 mM KH2PO4, 10 mM Tris-HCl, pH 7.4, mitochondrial protein concentration 0.2 mg/ml) [48]. Fluorescence excitation at 540 nm; M540 fluorescence spectrum maximum, 585 nm; monochromator slits for excitation and emission, 6 × 5 nm. Curves 1-7: MbO2 concentrations of 0, 3.8, 7.5, 11.1, 17.9, 24.6, and 31 µM, respectively. b) Dependence of the corrected M540 fluorescence at the spectrum maximum on the MbO2 concentration (pH 7.4). Insert, estimation of the MbO2 binding constant with mitochondria in the quenching complex.
Fig. 6. Proposed localization of merocyanine M540 fluorescent probe in a bilayer phospholipid membrane [48, 59].
Table 2. Fluorescence quenching of
mitochondria-bound lipid probe merocyanine 540 by oxymyoglobin and
metMb at pH 6-8 at different ionic strength values (10-150 mM
KCl in 10 mM Tris-HCl buffer) [48]
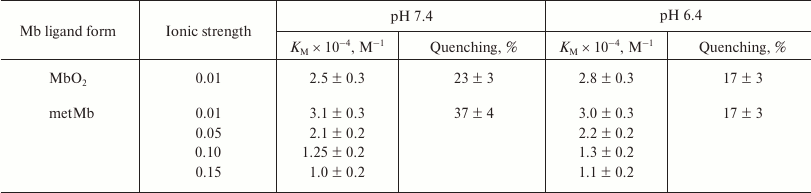
At low ionic strength, the constant for metMb binding to mitochondria was ~1.5 times higher than for MbO2. It decreased with an increase in the ionic strength within the 10-150 mM interval of KCl concentration (Table 2). At the same time, the KM values for each of the quenchers (determined at pH 6.4 and 7.4) did not differ within the pH range of 6-8, i.e. the total protein charge, which changes in this pH interval, did not affect MbO2 and metMb binding to the mitochondrial membrane. Therefore, the effect of ionic strength on the affinity of myoglobin for mitochondria must be due to local electrostatic interactions.
The difference in the binding of MbO2 and metMb to the membrane of Mch could not be explained by the ligand-induced conformational changes in the protein structure, since such changes are insignificant in many ligand-bound myoglobins and take place mostly at the ligand-binding site in the distal part of the heme pocket [4, 5]. It is possible that this difference is related to differences in the electronic state and charge of the heme complexes, namely, between the diamagnetic neutral ferroheme in MbO2 (O2 as a ligand of the Fe atom) and paramagnetic charged ferriheme in metMb (+1 charge; water molecule as a ligand of the Fe atom). Hence, charged amino acid residues near the Mb heme pocket, whose ionization state does not change within the pH interval 6-8, interact most probably with the phospholipids of the membrane.
Interaction of myoglobin with artificial phospholipid bilayer membranes (BLMs). Artificial BLMs are an excellent model of real biological structures and are widely used for studying biological processes that involve membranes. The data on the interaction of Mb with mitochondria obtained using fluorescence methods correlate well with the results of studies of the interaction of MbO2 and metMb with artificial BLMs using potentiodynamic method, also known as the method of capacity minimization. The adsorption of MbO2 and metMb on either cis- or trans-surface of the BLM, to which regulated constant sinusoidal voltage is applied, was studied by measuring the difference of potentials between the cis- and trans-surfaces of BLM (ΔE, mV) resulting from Mb adsorption on one of these surfaces [49, 64]. To modulate Mb interaction with the negatively charged mitochondrial membrane, BLM was produced from the negatively charged phospholipid POPG or neutral soybean lecithin (phosphatidylcholine).
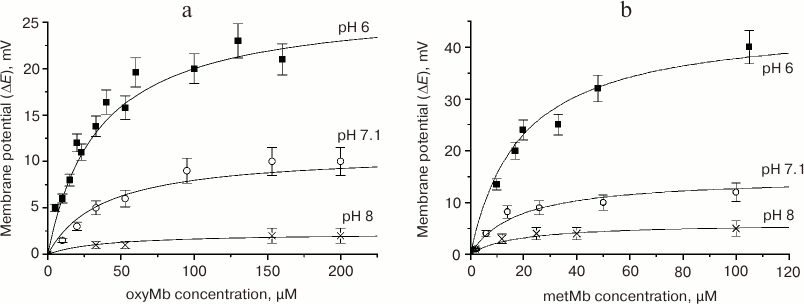
It was found that MbO2 and metMb interacted with both neutral lecithin BLM and negatively charged BLM from POPG (Fig. 7, a and b). The dependence of ΔE on the concentration of MbO2 and metMb had the shape typical for the Langmuir adsorption isotherm and could be described by the equation:
where c is Mb concentration, A = 1/α is absorption activity of the protein that characterizes MbO2 and metMb affinity for BLM.
Fig. 7. Dependence of the difference in potentials of the bilayer lecithin membrane (ΔE) on the concentration of MbO2 (a) and metMb (b) at the trans-side of the membrane [49]. Experimental conditions: 20 mM KCl, 15 mM Tris-maleate buffer. Solid lines, calculated Langmuir isotherms: a) ΔEmax = 27 mV, α = 36 µM (at pH 6.0); ΔEmax = 11 mV, α = 38 µM (at pH 7.1); ΔEmax = 2.3 mV, α = 40 µM (at pH 8.0); b) ΔEmax = 45 mV, α = 19 µM (at pH 6.0); ΔEmax = 15 mV, α = 18 µM (at pH 7.1); ΔEmax = 6 mV, α = 18 µM (at pH 8.0).
The saturation potential, at which the curve reaches the plateau (ΔEmax), is directly proportional to the surface charge density and relates to the number of protein molecules adsorbed at the BLM surface and to the area occupied by these molecules. The calculations showed that at ΔE = ΔEmax, the membrane surface was covered with myoglobin monolayer [49]. The differences in ΔEmax within the pH 6-8 interval were related to different total charges of the adsorbed protein. The maximal positive charge was observed at pH 6; the minimal (close to 0) charge was observed at pH 8.0; pH 7.1 was characterized by the intermediate ΔEmax value. For each protein, the saturation potentials at the BLMs composed of lecithin and POPG were the same at the same pH values, since they were determined solely by the protein molecule charge. The saturation potentials for metMb would be 1.5-2 times higher than for MbO2 at the same pH value, because metMb has an additional positive charge on the heme Fe atom.
The parameter α is the protein concentration at which the potential equals ΔEmax/2. The adsorption of both proteins on the negatively charged POPG membrane was more efficient than on the lecithin BLM because saturation was reached at lower protein concentrations (Table 3). The affinities of metMb and MbO2 toward the POPG membrane were ~2.5 and ~15 times higher, respectively, than toward the lecithin membrane.
Table 3. Parameters of MbO2 and
metMb adsorption on the neutral lecithin BLM and negatively charged BLM
from POPG [49]
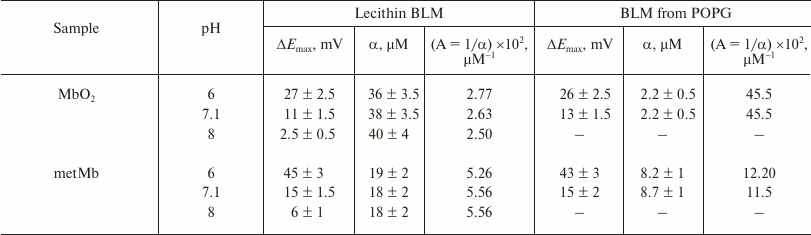
The parameter α for each of the proteins adsorbed on the lecithin and POPG BLMs was virtually the same at pH 6-8 (Table 3), i.e. the affinities of MbO2 and metMb toward the membrane did not depend on pH and were independent of the total protein charge that changed within this pH interval. At the same time, metMb ~2.5 times more efficiently bound to the lecithin BLM, while MbO2 ~3.5 times more efficiently bound to the POPG membrane. As mentioned above, conformational differences between these proteins involve mainly the heme region; therefore, we suggest that in this case also local electrostatic interactions took place between anionic groups (heads) of BLM phospholipids and Mb cationic groups near the heme, most probably, Lys and Arg residues surrounding the heme pocket.
Therefore, MbO2 and metMb adsorption on phospholipid membranes is mostly of electrostatic nature, since its efficiency significantly increased for the negatively charged POPG membrane. The total charge of the Mb molecule, that changes within the pH 6 to 8 interval, only affects the potential on the membrane, but not the affinity of MbO2 and metMb to the membrane (Table 3).
Effect of artificial and natural phospholipid membranes on the conformation of Mb and its affinity for oxygen. Conformational changes in myoglobin induced by its interaction with phospholipid membranes. It has become evident that conformation, stability, and even functional properties of water-soluble proteins in different cell compartments significantly differ from the same properties in solution. The main reason is that in cells, proteins interact with numerous charged polymeric structures, primarily with membranes. Electrostatic and hydrophobic interactions with the membrane surface might change protein conformations that determine protein interaction with other protein and complexes, translocation across the membranes, oligomerization, and protein functions, such as electron and ligand transport, enzymatic catalysis, etc. Despite small positive charge of Mb, electrostatic interactions near negatively charged membrane surface might be amplified due to the local decrease in the effective dielectric permittivity and medium pH [65, 66].
Conformational changes in sperm whale metMb in the presence of liposomes (vesicles) composed of negatively charged phospholipids POPG and DPPG at different phospholipid/metMb ratios and pH values have been extensively studied by absorption spectroscopy, circular dichroism (CD) spectroscopy, high-resolution 1H-NMR, scanning microcalorimetry, proteolysis, and other physicochemical methods [67, 68].
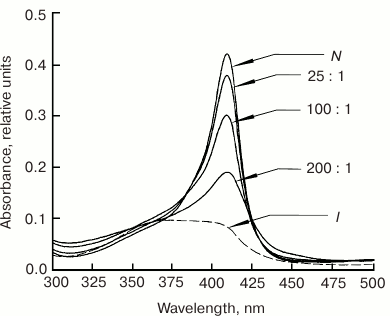
It was found that at the minimal POPG/metMb ratio of 25 : 1 (pH 7.2), the intensity of the Soret band in the metMb absorption spectrum decreases (Fig. 8), which indicates conformational changes near the heme. As the phospholipid concentration increases, the spectrum changes even more – the maximum broadens and shifts to shorter wavelength, thereby suggesting disturbances in the heme pocket native stricture due to the break of the coordination bond between the Fe atom and proximal His93(F8). According to our data (not published), liposomes composed of neutral lecithin produce considerably less effect on the heme surroundings in metMb, since changes in the intensity of the Soret band (with preservation of the spectrum shape) were observed only at lecithin/metMb ratios of 200 : 1 and higher.
Fig. 8. Absorption spectra of sperm whale metMb in the Soret band in the presence of negatively charged POPG liposomes at different POPG/metMb molar ratios (shown next to the corresponding curves) in 10 mM Tris-HCl buffer, pH 7.2 [67]. Spectra of metMb in the native (N, pH 7.2) and intermediate (I, pH 3.6) states are shown for comparison.
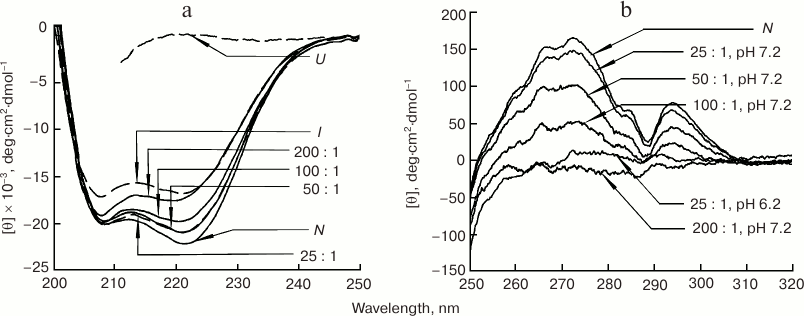
In the presence of 20-50-fold excess of POPG (pH 7.2), the CD spectrum of metMb in the far-UV spectral region with two minima at 208 and 220 nm, which is inherent for α-helical proteins, very closely resembled the CD spectrum of the native protein (Fig. 9a), i.e. secondary α-helical structure was preserved in metMb. An increase in the liposome content to 100-fold excess of POPG over metMb was accompanied by a decrease in the molar ellipticity at 220 nm with the preservation of the spectral shape. Nevertheless, all CD spectra in the presence of POPG liposomes strongly differed from the CD spectrum of unfolded metMb, which indicates that the protein retained a pronounced secondary structure even if it differed from the structure of the native metMb, which might be related to conformational changes caused by the interactions of the protein with the phospholipids.
Fig. 9. Far-UV (a) and near-UV (b) CD spectra of sperm whale metMb in the presence of negatively charged POPG liposomes at different POPG/metMb molar ratios (shown next to the corresponding curves). a) metMb spectra in the native (N), intermediate (I, pH 3.6), and completely unfolded (U, 6 M GuHCl) states are shown for comparison. b) N, spectrum of metMb in the native state at pH 7.2. Protein concentration, 0.5-1.0 mg/ml; 10 mM Tris-HCl, pH 7.2.
The CD spectrum of metMb in the near-UV spectral region in the presence of 25-fold molar excess of POPG (pH 7.2) only slightly differed from the native protein spectrum (Fig. 9b), thereby indicating that the spatial organization of the side groups in metMb was preserved. As concentration of POPG increased, rigid tertiary structure became partially disturbed (as seen from the ellipticity decrease with the preservation of the spectrum shape) and completely disappeared only at POPG/metMb ratio of 200 : 1 (amplitude of all characteristic bands in the spectrum became close to 0).
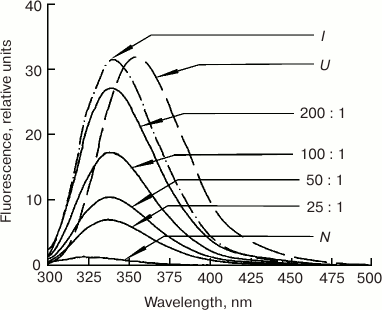
In the presence of 25-fold molar excess of POPG (pH 7.2), fluorescence of the two invariant tryptophan residues in the A-helix of Mb (Trp7(A5) and Trp14(A12)) was excited with a shift in the spectrum maximum to longer wavelength (Fig. 10). Since according to the Soret and CD spectra in the UV and visible regions, secondary and the tertiary structures of metMb were preserved under these conditions, we cannot exclude the influence of the hydrophobic phospholipid membrane of liposomes with which metMb interacted. The observed maximum of the metMb fluorescence spectrum (338 nm) was between the maxima typical for the native (325 nm) and completely unfolded (355 nm) structures, which might be related to incomplete exposure of tryptophan residues to water and indicate the presence of relatively compact metMb structure. The fluorescence increased with further increase in the POPG/metMb ratio, but the spectrum shape remained the same.
Fig. 10. Dependence of tryptophan fluorescence of metMb on concentration of phospholipids at different POPG/metMb molar ratios. Spectra of metMb in the native (N), intermediate (I, pH 3.6), and completely unfolded (U, 6 M GuHCl) states are shown for comparison [67]. Excitation wavelength, 293 nm; protein concentration, 0.5 mg/ml; 10 mM Tris-HCl, pH 7.2.
According to data of optical spectrometry, CD spectroscopy in near- and far-UV spectral regions, and fluorescence spectrometry, similar changes in the metMb conformation also took place at pH 6.2, but at much lesser phospholipid concentration, thereby showing an important role of electrostatic forces in the Mb–membrane interactions. Thus, already in the presence of 25-fold excess of POPG, the Soret band changed its shape and metMb lost the tight packing of its side groups. The data obtained by high-resolution 1H-NMR, scanning microcalorimetry, proteolysis, and other methods also suggest the metMb conformational stability is disturbed in the presence of POPG and DPPG liposomes [67, 68].
Therefore, the interaction of Mb with negatively charged phospholipid membranes at physiological pH results in the changes in the protein conformation, primarily the conformation of the heme pocket (even at the lowest phospholipid concentrations), that occur without any noticeable disturbances in the secondary and tertiary structures of the protein. Increase in the phospholipid concentration and decrease in pH significantly promote conformational changes in Mb, probably via strengthening interactions of Mb with the membrane.
Effect of artificial BLMs and mitochondrial membranes on the autooxidation rate of oxymyoglobin. Conformational changes in the heme pocket should in turn affect the affinity of myoglobin for O2. Even small changes in the heme pocket structure in monomeric globins with highly homologous tertiary structures result in significant (ten and hundred times) differences in the kinetic and equilibrium parameters of the ligand binding [4, 5]. Under aerobic conditions, changes in the affinity of Mb for oxygen could be detected by changes in the rate of MbO2 autooxidation (kox) (spontaneous conversion into metMb), since it was experimentally demonstrated that the rate of MbO2 autooxidation directly correlates with the equilibrium constant of O2 dissociation (Kdis) [32, 69].
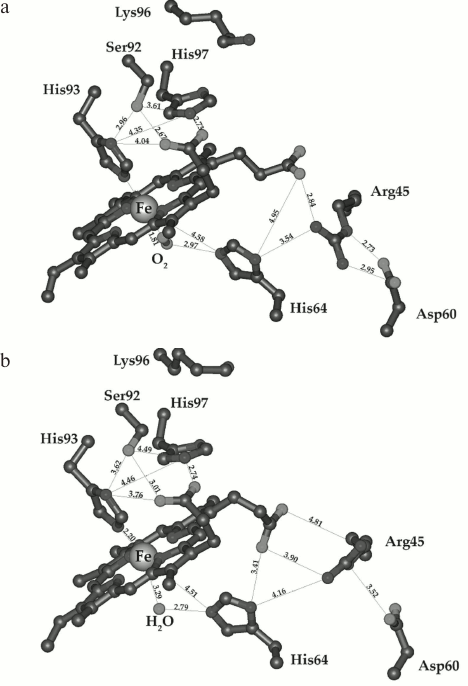
Direct correlation between kox and Kdis suggests that both processes are regulated by the same structural properties of the protein, primarily by the conformation of the heme pocket and the presence of a hydrogen bond between the liganded O2 and the distal His64(E7) (Fig. 11, a and b). This hydrogen bond ensures high stability of the heme oxygen complex in MbO2 by preventing dissociation of the bound O2. At the same time, it prevents MbO2 autooxidation, which is also initiated by the break of the H-bond with subsequent protonation of the FeO2 complex and superoxide dissociation (rate-limiting stage) [32, 69]. Substitution of His64(E7) in the heme pocket with nonpolar amino acids incapable of H-bonding increases MbO2 autooxidation rate 100-800-fold [69].
Fig. 11. Structures of the heme pocket distal region in sperm whale oxymyoglobin (a) and metMb (b). Coordinates of atoms were taken from the PBD 1A6M and 1VXA database and visualized with MolMol program software. The distances between atoms are shown in angstroms [50].
Autooxidation of the sperm whale MbO2 is an extremely slow reaction that takes several days at neutral pH values at room temperature and can be significantly accelerated by lowering pH and increasing temperature [32, 69].
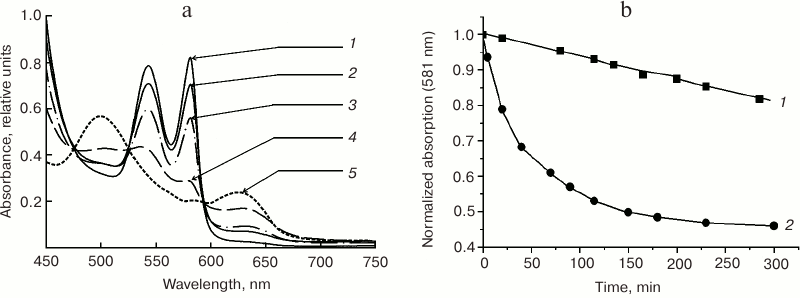
The effect of liposomes composed from neutral lecithin or negatively charged phospholipid POPG was studied at different phospholipid/MbO2 ratios and different temperatures (pH 7.2) [50]. In the absence of liposomes, the characteristic spectrum of MbO2 did not undergo any significant changes within 3.5 h of incubation. In the presence of 25-fold molar excess of POPG (pH 7.2, 37°C), a considerable portion of MbO2 oxidized into metMb after 40 min of incubation; after 6 h of incubation, all the MbO2 was converted into metMb (Fig. 12, a and b), i.e. the rate of MbO2 autooxidation increased almost 20 times. At 100-fold excess of POPG, MbO2 was converted into metMb almost immediately (Fig. 12a, curve 5).
Fig. 12. a) Changes in the visible absorption spectrum of sperm whale MbO2 in the presence of 25-fold excess of negatively charged POPG liposomes in 10 mM Tris-HCl (pH 7.2) at 37°C [50]. Curves: 1) MbO2 spectrum in the absence of liposomes; 2) same as (1) after 3.5 h of incubation; 3) MbO2 spectrum 40 min after addition of 25-fold molar excess of POPG; 4) same as (3) after 6 h of incubation; 5) MbO2 spectrum after addition of 100-fold molar excess of POPG. b) Effect of negatively charged POPG liposomes on the rate of oxymyoglobin autooxidation in 10 mM Tris-HCl (pH 7.2) at 37°C [50]. Curves: 1) kinetics of MbO2 oxidation in the absence of liposomes; 2) kinetics of MbO2 oxidation in the presence of 25-fold molar excess of negatively charged POPG liposomes.
The process slows at room temperature, which allowed comparison of the kinetics of MbO2 autooxidation in the presence of the liposomes composed from neutral lecithin and negatively charged POPG at different phospholipid/protein molar ratios. The effect of negatively charged liposomes on kox was significantly greater than that of neutral lecithin liposomes. This correlated well with the potentiodynamic data showing that adsorption of MbO2 on the POPG BLM is more efficient than on the lecithin BLM [49]. Thus, the MbO2 autooxidation rate in the presence of 50-fold and 100-fold excesses of lecithin increased 8 and 25 times, respectively, as compared to the control, while 25-fold and 100-fold excesses of POPG accelerated the same reaction 22 and 174 times, respectively. This shows that the rate of MbO2 autooxidation in the presence of 25-fold excess of POPG is approximately three times higher than in the presence of 50-fold excess of lecithin. At the 100 : 1 phospholipid/protein ratio, the MbO2 autooxidation rate in the presence of POPG liposomes is seven times higher than in the presence of lecithin liposomes.
The rate of MbO2 autooxidation also increases in the presence of antimycin A-inhibited rat liver mitochondria in a low-ionic-strength medium (~10- and 20-fold at the mitochondria concentration of 1 and 2 mg protein/ml mitochondrial suspension, respectively) [50]. Increase in the ionic strength to 110 mM KCl at the 1 mg/ml mitochondrial protein concentration was accompanied by 2-fold decrease in kox. The fact that the rate of MbO2 autooxidation is proportional to the concentration of Mch in the suspension and depends on the ionic strength provides additional evidence that this reaction is determined by the interaction of Mb with mitochondria and electrostatic forces play an important role in this process.
It should be noted that the stabilization of the native conformation of the heme pocket in MbO2 is achieved mainly due to hydrophobic interactions between the heme complex and nonpolar protein groups in the heme surroundings and the hydrogen bond between the distal His64(E7) and liganded O2. But very important also are electrostatic interactions between heme-1-propionate and His97(FG2) and between heme-6-propionate, Arg45(CD3), Asp60(E3), and His64(E7), which stabilize the position of the distal His64(E7) as the hydrogen donor to O2 (Fig. 11, a and b). The substitution of Lys45 in pig MbO2 (analog of Arg45 in sperm whale Mb) with small noncharged Ser increases the MbO2 autooxidation rate 7-fold, while substitution of the same residue with Glu drastically (~20 times) increases kox. Apparently, the loss of the positive charge at position 45 or introduction of the negative charge (Glu) at this position disturb the native structure of this protein fragment and increase the accessibility of the distal region of the heme pocket to the solvent [69].
Electrostatic interactions between MbO2 and negatively charged groups of natural and artificial phospholipid membranes might underlie similar disturbances in the native structure of the heme pocket. Under aerobic conditions, these changes result in the acceleration of MbO2 autooxidation, which is equivalent to the decrease in the Mb affinity for the ligand and should facilitate O2 dissociation at physiological pO2 values.
Effect of liposomes and mitochondrial membranes on the oxy/deoxyMb equilibrium under anaerobic conditions. The affinity of Mb toward oxygen in myocytes is 4-5-fold lower than in solution at 37°C – a fact that has been noted by many authors [5]. However, as mentioned above, no compounds that can noticeably affect the Mb p50 value have been found so far. Moreover, it was shown that Mb p50 value in rat myocytes depends on the presence and state of mitochondria [12].
Because of the high affinity of Mb for O2, it is difficult to estimate the changes in the ratio between oxy- and deoxy-forms of Mb in a solution under aerobic condition, since the equilibrium in the Mb(2) reaction with oxygen will be almost completely shifted toward MbO2 formation (Eq. (1)). Therefore, changes in the MbO2/Mb(2) ratio in a solution in the presence of artificial BLMs or mitochondrial membranes were monitored under anaerobic conditions, when O2 concentration in the MbO2 solution is close to zero [51, 70, 71]. Oxygen was eliminated from the MbO2 solution in a closed polarographic cell using respiring mitochondria placed in a sealed dialysis bag that was impenetrable for proteins (no MbO2 deoxygenation was observed) (Fig. 2a), and then lecithin liposomes or antimycin A-inhibited rat liver mitochondria were added to the resulting anaerobic MbO2 solution. In both cases, absorbance at 542 and 581 nm (typical for MbO2) decreased, while absorbance at 560 nm (Mb(2) absorbance maximum) increased [70].
The shift in the oxy/deoxyMb equilibrium toward the ligand-free Mb(2) might be related to the interaction of the protein with the phospholipid membrane. According to our data, p50 for Mb in a mitochondrial suspension at 25°C was ~5 mm Hg [46] (~6.5 mm Hg according to Tang et al. [57]), i.e. interaction of Mb with mitochondrial membrane phospholipids decreased Mb affinity to O2 10-12 times, which considerably facilitated O2 dissociation from Mb at physiological pO2 values in a cell.
CONCLUSION
Oxygen dissociation from MbO2 at physiological O2 concentrations takes place only upon active interaction of MbO2 with the mitochondrial membrane. The result of this interaction is a decrease in the affinity of Mb for O2 due to the changes in the heme pocket conformation. An important role in the interaction between Mb and mitochondria belongs to the Coulomb electrostatic interactions between positively charged Lys and Arg residues in the heme surroundings and negatively charged phospholipid groups (heads) of the membrane.
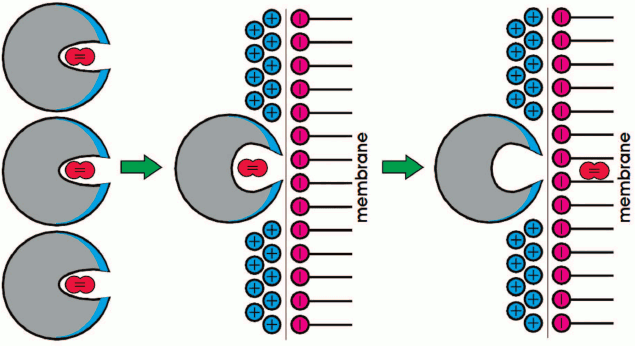
In the presence of respiring mitochondria, the solution should contain at least two types of Mb molecules: free molecules with high affinity for O2 and, correspondingly, low p50 value, and mitochondria-bound Mb molecules with low affinity for O2 and higher p50 value:
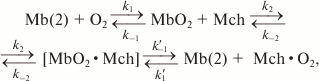 (4)
(4)where k1 and k–1 are constants for O2 binding to and dissociation from Mb in a solution, respectively; k1′ and k–1′ are the same constants, but for the complex of myoglobin with mitochondria, and k2 and k–2 are the constants of the formation and dissociation of the Mb–mitochondria complex. The difference between k1′ and k–1′ and the corresponding constants without the apostrophes is due to the changes in the heme pocket conformation caused by the interaction of Mb with the mitochondrial membrane (Fig. 13).
Fig. 13. Scheme of MbO2 function in a myocyte [46].
To ensure efficient O2 transfer at low pO2 values, Mb should not form a stable complex with the mitochondrial membrane, and the exchange between the two types of MbO2 molecules (free and membrane-bound) should occur very fast. For example, it must occur at the rate of rotational diffusion of Mb that changes orientation of the heme pocket relative to the mitochondrial surface. In the opposite case, myoglobin would not be able to efficiently accelerate O2 transport to the mitochondria under oxygen deficit conditions. Note that the rate of rotational diffusion of Mb in myocytes is only 1.5-fold lower than in dilute aqueous solutions [9, 11, 13, 14].
Therefore, unlike current mathematical models for estimating the contribution of Mb to the cell supply with oxygen that assume that Mb affinity for oxygen remains constant (one pair of association/dissociation constants), the calculations should use three different pairs of constants (Eq. (4)). The observed constants for the MbO2 binding with mitochondria (~104 M–1 at I = 0.15 M) and the lifetime of the complex (tens of nanoseconds) correspond to a low affinity of Mb toward the mitochondrial membrane, which is optimal for the efficient functioning of Mb as an oxygen transporter.
REFERENCES
1.Hill, R. L. (1933) Oxygen affinity of muscle
haemoglobin, Nature, 132, 897-898.
2.Millikan, G. A. (1939) Muscle hemoglobin,
Physiol. Rev., 19, 503-523.
3.Kendrew, J. C., Dickerson, R. T., Strandberg, B.
E., Hart, R. G., Davis, D. R., Phillips, D. C., and Shore, V. C. (1960)
Structure of myoglobin. A three-dimensional Fourier synthesis at 2
Å resolution, Nature (London), 185,
422-427.
4.Antonini, E., and Brunori, M. (1971) Hemoglobin
and Myoglobin in Their Reactions with Ligands, Front Biology,
Amsterdam-London.
5.Starodub, R. F., Korobov, V. N., and Nazarenko, V.
I. (1992) Myoglobin: Structure, Properties, Synthesis, and
Biological Role [in Russian], Naukova Dumka, Kiev.
6.Garry, D. J., and Mammen, P. P. (2007) Molecular
insights into the functional role of myoglobin, Adv. Exp. Med.
Biol., 618, 181-193.
7.Wittenberg, J. B. (1970) Myoglobin facilitated
oxygen diffusion and the role of myoglobin in oxygen entry into muscle,
Physiol. Rev., 50, 559-636.
8.Wittenberg, B. A., Wittenberg, J. B., and Caldwell,
P. R. B. (1975) Role of myoglobin in the oxygen supply to red skeletal
muscle, J. Biol. Chem., 250, 9038-9043.
9.Wittenberg, J. B., and Wittenberg, B. A. (1981)
Facilitated diffusion by oxygen carriers, in Oxygen and Living
Processes (Gilbert, D. L., ed.) Springer, New York, pp.
177-199.
10.Wittenberg, B. A., and Wittenberg, J. B.
(1989) Transport of oxygen in muscle, Ann. Rev. Physiol.,
51, 857-878.
11.Fletcher, J. E. (1980) On facilitated oxygen
diffusion in muscle tissues, Biophys. J., 29,
437-458.
12.Jones, D. P., and Kennedy, F. G. (1982)
Intracellular O2 gradients in cardiac myocytes. Lack of a
role for myoglobin in facilitation of intracellular O2
diffusion, Biochem. Biophys. Res. Comm., 105,
419-424.
13.Jelicks, I. A., and Wittenberg, B. A. (1995)
1H nuclear magnetic resonance studies of sarcoplasmic
oxygenation in the red cell-perfused rat heart, Biophys. J.,
68, 2129-2136.
14.Jurgens, K. D., Papadopoulos, S., Peters, T., and
Gross, G. (2000) Myoglobin: just an oxygen store or also an oxygen
transporter? News Physiol. Sci., 15, 269-274.
15.Whiteley, J. P., Gavaghan, D. J., and Hahn, C. E.
W. (2002) Mathematical modeling of oxygen transport to tissue, J.
Math. Biol., 44, 503-522.
16.Wittenberg, J. B., and Wittenberg, B. A. (2003)
Myoglobin function reassessed, J. Exp. Biol., 206,
2011-2020.
17.Tilakaratne, H. K., Hunter, S. K., and Rodgers,
V. G. J. (2002) Mathematical modeling of myoglobin facilitated
transport of oxygen in devices containing myoglobin –
expressing cells, Math. Biosci., 176, 253-267.
18.Chung, Y., and Jue, Th. (1999) Regulation
of respiration in myocardium in the transient and steady state, Am.
J. Physiol. Heart Circ. Physiol., 277, 1410-1417.
19.Sharonov, Yu. A., and Sharonova, N. A. (1975)
Structure and function of hemoglobin, Mol. Biol. (Moscow),
9, 145-172.
20.Papadopoulos, S., Endeward, V., Revesz-Walker,
B., Jurgens, K. D., and Gross, G. (2001) Radial and longitudinal
diffusion of myoglobin in single living heart and skeletal muscle
cells, Proc. Natl. Acad. Sci. USA, 98, 5904-5909.
21.Lin, P.-Ch., Kreutzer, U., and Jue, Th. (2007)
Myoglobin translational diffusion in rat myocardium and its implication
on intracellular oxygen transport, J. Physiol., 578,
595-603.
22.Garry, D. J., Ordway, G. A., Lorenz, J. N.,
Radford, N. B., Chin, E. R., Grange, R. W., Bassel-Duby, R., and
Williams, R. S. (1998) Mice without myoglobin, Nature,
395, 905-908.
23.Vinogradov, A. D. (1999) Myoglobin: what is it
for? Biochemistry (Moscow), 64, 592-593.
24.Skulachev, V. P. (1999) Comments for the article
“Myoglobin: what is it for?” by A. D. Vinogradov,
Biochemistry (Moscow), 64, 594.
25.Flogel, U., Merx, M. W., Godecke, A., Decking, U.
K., and Shrader, J. (2001) Myoglobin: a scavenger of bioactive NO,
Proc. Natl. Acad. Sci. USA, 98, 735-740.
26.Merx, M. W., Godecke, A., Flogel, U., and
Shrader, J. (2005) Oxygen supply and nitric oxide scavenging by
myoglobin contribute to exercise endurance and cardiac function,
FASEB J., 19, 1015-1017.
27.Flogel, U., Godecke, A., Klotz, L. O., and
Shrader, J. (2004) Role of myoglobin in the antioxidant defense of the
heart, FASEB J., 18, 1156-1158.
28.Brunori, M. (2001) Nitric oxide, cytochrome
c oxidase and myoglobin, Trends Biochem. Sci., 26,
21-23.
29.Doeller, J. E., and Wittenberg, B. A. (1991)
Myoglobin function and energy metabolism of isolated cardiac myocytes:
effect of sodium nitrite, Am. J. Physiol., 261,
53-62.
30.Jourd’heuil, D., Mills, L., Miles, A. M.,
and Grisham, M. B. (1998) Effect of nitric oxide on
hemoprotein-catalyzed oxidative reactions, Nitric Oxide,
2, 37-44.
31.Sievers, G., and Ronnberg, M. (1978) Study of the
pseudoperoxidatic activity of soybean leghemoglobin and sperm whale
myoglobin, Biochim. Biophys. Acta, 533, 293-301.
32.Shikama, K. (1998) The molecular mechanism of
autoxidation for myoglobin and hemoglobin: a venerable puzzle, Chem.
Rev., 98, 1357-1373.
33.Gunther, M. R., Sampath, V., and Caughey, W. S.
(1999) Potential roles of myoglobin autoxidation in myocardial
ischemia-reperfusion injury, Free Radic. Biol. Med., 26,
1388-1395.
34.Gloster, J., and Harris, P. (1977) Fatty acid
binding to cytoplasmic proteins of myocardium and red and white
skeletal muscle in the rat. A possible new role for myoglobin,
Biochem. Biophys. Res. Comm., 74, 506-513.
35.Yackzan, K. S., and Wingo, W. J. (1982) Transport
of fatty acids by myoglobin – a hypothesis, Med.
Hypotheses, 8, 613-618.
36.Gotz, F. M., Hertel, M., and Groschel-Stewart, U.
(1994) Fatty acid binding of myoglobin depends on its oxygenation,
Biol. Chem. Hoppe Seyler, 375, 387-392.
37.Sriram, R., Kreutzer, U., Shih, L., and Jue, T.
(2008) Fatty acid binding to myoglobin, FEBS Lett., 582,
3643-3649.
38.Godecke, A., Flogel, U., Zanger, K., Ding, Zh.,
Hirchenhain, J., Decking, U. K. M., and Schrader, J. (1999) Disruption
of myoglobin in mice induces multiple compensatory mechanisms, Proc.
Natl. Acad. Sci. USA, 96, 10495-10500.
39.Grange, R. W., Meeson, A., Chin, E., Lau, K. S.,
Stull, J. T., Shelton, J. M., Williams, S. R., and Garry, D. J. (2001)
Functional and molecular adaptations in skeletal muscle of
myoglobin-mutant mice, Am. J. Physiol. Cell Physiol.,
281, 1487-1494.
40.Meeson, A. P., Radford, N., Shelton, J. M.,
Mammen, P. P. A., DiMaio, J. M., Hutcheson, K., Kong, Y., Elterman, J.,
Williams, R. S., and Garry, D. J. (2001) Adaptive mechanisms that
preserve cardiac function in mice without myoglobin, Circ. Res.,
88, 713-720.
41.Schlieper, G., Kim, J.-H., Molojavyi, A., Jacoby,
Ch., Laussmann, T., Flogel, U., Godecke, A., and Schrader, J. (2004)
Adaptation of the myoglobin knock out mouse to hypoxic stress, Am.
J. Physiol. Regul. Integr. Comp. Physiol., 286, 786-792.
42.Pesce, A., Bolognesi, M., Bocedi, A., Ascenzi,
P., Dewide, S., Moens, L., Hankeln, T., and Burmester, T. (2002)
Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin
family, EMBO Rep., 3, 1146-1151.
43.Riggs, A. F., and Gorr, T. A. (2006) A globin in
every cell? Proc. Natl. Acad. Sci. USA, 103,
2469-2470.
44.Burmester, T., Gerlach, F., and Hankeln, T.
(2007) Regulation and role of neuroglobin and cytoglobin under hypoxia,
Adv. Exp. Med. Biol., 618, 169-180.
45.Fraser, J., Vieira de Mello, L., Ward, D., Rees,
H. H., Williams, D. R., Fang, Y., Brass, A., Gracey, A. Y., and
Cossing, A. R. (2006) Hypoxia-inducible myoglobin expression in
nonmuscle tissues, Proc. Natl. Acad. Sci. USA, 103,
2977-2981.
46.Postnikova, G. B., and Tselikova, S. V. (2005)
Myoglobin and mitochondria: kinetics of oxymyoglobin deoxygenation in a
mitochondrial suspension, Biophysics (Moscow), 50,
284-291.
47.Postnikova, G. B., Tselikova, S. V., and
Shekhovtsova, E. A. (2009) Myoglobin and mitochondria: oxymyoglobin
interacts with mitochondrial membrane during deoxygenation,
Biochemistry (Moscow), 74, 1211-1218.
48.Postnikova, G. B., and Shekhovtsova, E. A. (2012)
Fluorescence studies on the interaction of myoglobin with mitochondria,
Biochemistry (Moscow), 77, 280-287.
49.Grigoriev, P. A., Postnikova, G. B., and
Shekhovtsova, E. A. (2012) Study of the interaction of myoglobin with
lipid bilayer membranes by potentiodynamic method, Biophysics
(Moscow), 57, 55-60.
50.Postnikova, G. B., and Shekhovtsova, E. A. (2013)
Effect of artificial and natural phospholipid membranes on rate of
sperm whale oxymyoglobin autooxidation, Biochemistry (Moscow),
78, 267-272.
51.Postnikova, G. B., and Shekhovtsova, E. A. (2015)
The effect of mitochondrial and artificial bilayer phospholipid
membranes on conformation of myoglobin and its affinity for oxygen,
Am. J. Biol. Chem., 3, 16-32.
52.Postnikova, G. B., Tselikova, S. V.,
Ignat’ev, D. A., and Kolaeva, S. G. (1997) Seasonal changes in
myoglobin content in muscles of hibernating yakutian ground squirrels,
Biochemistry (Moscow), 62, 141-144.
53.Postnikova, G. B., Tselikova, S. V., Kolaeva, S.
G., and Solomonov, N. G. (1998) Why high muscle myoglobin level is
required in hibernating ground squirrels? Dokl. Akad. Nauk,
360, 697-698.
54.Postnikova, G. B., Tselikova, S. V., Kolaeva, S.
G., and Solomonov, N. G. (1999) Myoglobin content in skeletal muscles
of hibernating ground squirrels rises in autumn and winter, Comp.
Biochem. Physiol., Part A, 124, 35-37.
55.Wittenberg, J. B., and Wittenberg, B. A. (2007)
Myoglobin-enhanced oxygen delivery to isolated cardiac mitochondria,
J. Exp. Biol., 210, 2082-2090.
56.Yamada, T., Furuichi, Y., Takakura, H.,
Hashimoto, T., Hanai, Y., Jue, Th., and Masuda, K. (2013) Interaction
between myoglobin and mitochondria in rat skeletal muscle, J. Appl.
Physiol., 114, 490-497.
57.Tang, J., Faustman, C., Hoagland, Th. A.,
Mancini, R. A., Seyfert, M., and Hunt, M. C. (2005) Mitochondrial
reduction of metmyoglobin: dependence on the electron transport chain,
J. Agric. Food Chem., 53, 5449-5455.
58.Shuruta, S. A., Amerkhanov, Z. G., and
Postnikova, G. B. (1999) Effect of mitochondria on the redox reaction
between oxymyoglobin and ferricytochrome c, Biophysics
(Moscow), 44, 1023-1026.
59.Vladimirov, Yu. A., and Dobretsov, G. E. (1980)
Fluorescent Probes in the Studies of Biological Membranes [in
Russian], Nauka, Moscow.
60.Jensen, L. J., Kuhn, M., Stark, M., Chaffron, S.,
Creevey, C., Muller, J., Doerks, T., Julien, P., Roth, A., Simonovic,
M., Bork, P., and Von Mering, C. (2009) Protein data bank, Nucleic
Acids Res., 37, 412-416.
61.Postnikova, G. B. (1999) Fluorescence study of
conformational transitions in the structure of myoglobin,
Biochemistry (Moscow), 64, 267-286.
62.Lelkes, P. I., and Miller, I. R. (1980)
Perturbations of membrane structure by optical probes: I. Location and
structural sensitivity of merocyanine 540 bound to phospholipid
membranes, J. Membr. Biol., 52, 1-15.
63.Verkman, A. S. (1987) Mechanism and kinetics of
merocyanine 540 binding to phospholipid membranes, Biochemistry,
26, 4050-4056.
64.Postnikova, G. B., and Shekhovtsova, E. A. (2014)
The interaction of myoglobin with neutral and negatively charged
artificial bilayer phospholipid membranes. Their effect on conformation
of myoglobin and its affinity for oxygen, Physiol. Sci.,
1, 1-11.
65.Bychkova, V. E., and Ptitsyn, O. B. (1993) The
molten globule in vitro and in vivo, Chemtracts
Biochem. Mol. Biol., 4, 133-163.
66.Ptitsyn, O. B. (1995) Molten globule and protein
folding, Adv. Protein Chem., 47, 83-229.
67.Basova, L. V. (2004) Effects of the Membrane
Surface on the Conformational State of Globular Proteins: Ph. D. in
Biology Dissertation, Institute of Cell Biophysics, Russian Academy of
Sciences, Pushchino.
68.Basova, L. V., Tiktopulo, E. I., Kutyshenko, V.
P., Klenin, S. I., Balobanov, V. A., and Bychkova, V. E. (2014)
Membrane-induced changes in the holomyoglobin tertiary structure:
interplay with function, Eur. Biophys. J., 43,
317-329.
69.Brantley, R. E., Smerdon, S. J., Wilkinson, A.
J., Singleton, E. W., and Olson, J. S. (1993) The mechanism of
autooxidation of myoglobin, J. Biol. Chem., 268,
6995-7010.
70.Postnikova, G. B., and Shekhovtsova, E. A. (2013)
Myoglobin and mitochondria: how does the “oxygen store”
work? J. Phys. Chem. Biophys., 3, 126.
71.Postnikova, G. B., and Shekhovtsova, E. A. (2014)
Myoglobin acts as the oxygen carrier to mitochondria, Physiol.
Sci., 1, 12-16.