Blood-Derived RNA- and microRNA-Hydrolyzing IgG Antibodies in Schizophrenia Patients
E. A. Ermakov1,2, S. A. Ivanova3, V. N. Buneva1,2, and G. A. Nevinsky1,2,a*
1Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, 630090 Novosibirsk, Russia2Novosibirsk State University, 630090 Novosibirsk, Russia
3Mental Health Research Institute, Tomsk National Research Medical Center, Russian Academy of Sciences, 634014 Tomsk, Russia
* To whom correspondence should be addressed.
Received September 7, 2017; Revision received December 26, 2017
Abzymes with various catalytic activities are the earliest statistically significant markers of existing and developing autoimmune diseases (AIDs). Currently, schizophrenia (SCZD) is not considered to be a typical AID. It was demonstrated recently that antibodies from SCZD patients efficiently hydrolyze DNA and myelin basic protein. Here, we showed for the first time that autoantibodies from 35 SCZD patients efficiently hydrolyze RNA (cCMP > poly(C) > poly(A) > yeast RNA) and analyzed site-specific hydrolysis of microRNAs involved in the regulation of several genes in SCZD (miR-137, miR-9-5p, miR-219-2-3p, and miR-219a-5p). All four microRNAs were cleaved by IgG preparations (n = 21) from SCZD patients in a site-specific manner. The RNase activity of the abzymes correlated with SCZD clinical parameters. The data obtained showed that SCZD patients might display signs of typical autoimmune processes associated with impaired functioning of microRNAs resulting from their hydrolysis by the abzymes.
KEY WORDS: abzymes, schizophrenia patients, polyribonucleotide, miRNA hydrolysisDOI: 10.1134/S0006297918050048
Abbreviations: AID, autoimmune disease; CC, correlation coefficient; MBP, myelin basic protein; MS, multiple sclerosis; SLE, systemic lupus erythematosus; SCZD, schizophrenia.
Schizophrenia (SCZD) is a chronic brain disorder and one of the
highest-priority issues in psychiatry [1]. SCZD
prevalence reaches approximately 1%. It is the most severe psychiatric
disorder in humans that is associated with disintegration of thinking
and emotional responsiveness [2]. SCZD is a
progressive disorder with a polymorphic clinical picture; it often
results in impaired social adaptation and ability to perform work. SCZD
is accompanied with impairments in synaptic transmission that result in
the neuronal damage leading to severe brain dysfunctions [3-5] that often occur in
utero or in early childhood [6].
Despite the fact that SCZD has been studied for a long time, its etiology and underlying pathogenetic mechanisms remain unclear. At the moment, there is no common theory of SCZD pathogenesis. Opposing points of view make it difficult to arrive to conclusions about pros or cons of various theories and add little to the understanding of the role that autoimmune response might play in SCZD [7, 8]. Nonetheless, a large body of evidence has demonstrated a link between SCZD and various immunological processes (see reviews [9-16]).
Analysis of single nucleotide polymorphisms in the genome-wide association studies revealed a close link between the human leukocyte antigen genes (HLA gene family), complement component 4, and other immune system genes located on chromosome 6 (6p21-p22) in SCZD patients [17-19]. Comparative meta-analysis of 62 studies showed elevated levels of pro-inflammatory cytokines in the blood serum and cerebrospinal fluid in a large cohort of SCZD patients as compared to their healthy counterparts [20]. The association between inflammation and schizophrenia was confirmed by the observed upregulation of the Toll-like receptor-triggered production of cytokines by immune cells in SCZD patients [21]. It was also found that pro-inflammatory cytokines activate indoleamine 2,3-dioxygenase and kynurenine monooxygenase in the tryptophan-kynurenine pathway, thereby causing accumulation of 3-hydroxykynurenine and the NMDA antagonist kynurenic acid [9], i.e., immunological disorders might affect glutamatergic, serotonergic, dopaminergic, and noradrenergic neurotransmission pathways [9-11].
Since 1980s, the ideas on the association between SCZD and autoimmune processes have been published and widely debated, although not yet commonly accepted. Impaired functioning of the immune system (including autoimmune responses) and dysregulation of immune cells in SCZD patients have been well documented [8, 13, 22-26].
Impairments in the central nervous system (CNS) in SCZD patients were found to be related to autoimmune processes resulting in the upregulated expression of autoantibodies against surface antigens of the CNS cells, a neuropsychiatric disorder known as autoimmune encephalopathy [22]. It is believed that the damage of the brain cell membranes in SCZD patients results in the exposure of self-antigens, and, as a consequence, production of antigen-specific autoantibodies [23-25]. Interestingly, anti-glutamate receptor antibodies were detected in patients with SCZD and other diseases, including AIDs [26]. Antibodies against NMDA-NR1, AMPA-GluR3, NMDA-NR2A, mGluR1, and mGluR5 were found in patients with SCZD, but also in patients suffering from encephalitis, epilepsy, systemic lupus erythematosus (SLE), neuropsychiatric SLE, cerebellar ataxia, Sjogren’s syndrome, mania, and stroke. Such anti-glutamate receptor autoantibodies can bind to neurons in several brains regions, activate glutamate receptors and downregulate their surface expression, activate endothelial cells of the blood–brain barrier, cause brain injury, disturb glutamate signaling and related functions, cause neuronal apoptosis, and induce psychiatric, behavioral, and cognitive abnormalities [26]. Several epidemiological studies reported that SCZD is associated with various AIDs. In particular, nine AIDs (thyrotoxicosis, celiac disease, acquired hemolytic anemia, interstitial cystitis, Sjogren’s syndrome, rheumatoid arthritis, type 1 diabetes, and myositis) have higher prevalence in SCZD patients than in the comparison group; 12 AIDs were detected at higher prevalence in parents of SCZD patients than in parents of SCZD patients [27]. An association between SLE and SCZD was observed in patients of different age, gender, and socioeconomic status [28]. SCZD was also considered as atypical SLE with prevailing psychiatric manifestations [29]. A subgroup of SCZD patients was found to exhibit signs of multiple sclerosis (MS), both disorders sharing similar etiology, while MS patients were discovered to have a higher risk of developing SCZD [30]. In some groups, association between SCZD and Hashimoto’s thyroiditis was shown, as well as elevated levels of autoantibodies against thyroglobulin and thyroid peroxidase [31-34]. Moreover, strong association between SCZD and pemphigus [35] and the presence of antiphospholipid antibodies in SCZD patients were documented [36]. Different subgroups of SCZD patients might display signs of various well-known AIDs. Therefore, identification of mechanisms underlying SCZD, including those involving autoimmune factors, is very important.
It has been demonstrated by now that SCZD pathogenesis involves multiple genes and their transcription products [37]. A number of studies were aimed at elucidating the role of microRNAs that are known to regulate as many as several hundreds of various genes [37-50]. Dysregulated expression of various microRNAs has been found in the blood serum [38, 39], peripheral blood mononuclear cells [40, 41], and various brain regions [42, 43] of SCZD patients. Strong association between the single nucleotide polymorphism in miR-137 and miR-9-5p and SCZD was observed [17, 18, 44]. miR-137 was found to play an important role in differentiation of embryonic stem cells, neuronal proliferation and differentiation, and synapse maturation [45]. By downregulating GluA1 subunit expression, miR-137 inhibits AMPA-receptor-mediated synaptic transmission [46, 47], influences neurotransmitter release from synaptic vesicles, and affects synaptic plasticity [48]. It also controls expression of the zinc finger protein 804A (ZNF804A) gene. ZNF804A, in its turn, inhibits expression of catechol-O-methyltransferase and dopamine receptor D2 [45], which results in impaired dopamine neurotransmission. Moreover, dopamine receptor D2 expression is also regulated by miR-9-5p [44]. miR-9-5p is involved in neuronal migration; expression of miR-9-5p is lowered in neural progenitor cells in SCZD patients [49]. miR-219 is essential for neural stem cell proliferation and neurodevelopment [50], oligodendrocyte differentiation, and axonal myelination [51].
Artificial catalytic antibodies (abzymes) raised against synthetic analogs of transition states have been well described [52-54]. It was found that natural autoantibodies can also display enzymatic activity. The emergence of these antibodies in the blood serum is a typical feature of various AIDs (see reviews [54-58]). Similarly to artificial abzymes [52-54], natural abzymes are antibodies raised against haptens mimicking transition states of catalytic reactions [54-58]. Moreover, anti-idiotypic antibodies against enzyme catalytic sites can also exhibit catalytic activity (see [54-58] and in-text citations).
Despite the fact that autoantibodies against DNA, RNA, and various proteins are found in the blood serum of healthy donors, such antibodies often possess no catalytic activity [54-58]. It was shown that in experimentally treated mice and patients with various AIDs, abzymes with DNase, protease, and amylase activities can serve as the earliest statistically significant markers of existing and developing autoimmune pathologies [59, 60]. Abzyme enzymatic activity might be detected even at the pre-disease stage, in the absence of overt AID markers and changes in proteinuria, when titers of antibodies against DNA, proteins, and other antigens still fall within the normal range. Therefore, abzyme enzymatic activity may be used as an indicative sign even at the pre-disease stage, as well as during the development of spontaneous and induced AIDs [54-60].
It was mentioned above that SCZD does not belong to typical AIDs. However, it was demonstrated recently that the blood serum from ~30% SCZD patients contains high amounts of anti-DNA antibodies (as compared to 37% in SLE patients); DNase [61] and MBP-hydrolyzing [62] activities were detected for the antibodies in the serum of 80-82% SCZD patients, thereby indicating the existence of pronounced autoimmune component in SCZD development.
Previously, we have detected RNA-hydrolyzing antibodies in the blood samples from patients with SLE, MS, polyarthritis, and autoimmune thyroiditis [63-66].
Considering the ability of catalytically active serum antibodies from AID patients to hydrolyze DNA and RNA, association between microRNAs and developing SCZD, and the role played by microRNAs in proliferation, differentiation, and maturation of neuronal cells, here we examined the RNA-hydrolyzing activity toward various RNA substrates and microRNAs of antibodies isolated from the blood samples of SCZD patients.
MATERIALS AND METHODS
Materials. All proteins, reagents, and sorbents were from Sigma or GE Healthcare (USA).
Patients. Blood serum samples collected from 35 SCZD patients (ages from 19 to 61; mean age, 39.0 ± 11.2 years; 12 males and 23 females) were used to purify antibodies. Clinical diagnosis was verified for all the patients and validated in accordance with the PANSS, AIMS and CGI International Psychometric Criteria (http://www.psychiatry.ru/stat/780). The patients were divided into three groups displaying positive (n = 16), negative (n = 18), and positive-negative (n = 1) PANSS symptoms (see [67-71] and http://www.psychiatry.ru/stat/77).
The patients in this study were diagnosed mostly with paranoid schizophrenia (ICD-10 code – F20.0, 30 patients) and simple-type schizophrenia (ICD-10 code – F20.6, five patients). Four out of 30 patients with paranoid schizophrenia were subtyped as F20.00 (paranoid), 13 as F20.01 (hebephrenic), 7 as F20.02 (catatonic), and 6 as F20.09 (unspecified).
Patients with positive symptoms had irregular thoughts and speech, delusions, and tactile, olfactory, auditory, and gustatory hallucinations, commonly interpreted as psychotic manifestations [67, 68]. Hallucinations were usually related to delusions [69].
Negative symptoms included loss or lack of normal personality traits and human capabilities, e.g., depression, deficit of normal emotional responses or other mental processes, poverty of speech, inability to feel pleasure, no wish to form new relationships, and lack of motivation. Such patients are less susceptible to medicated therapy [67, 70]. It is believed that negative symptoms contribute more to the decrease in the quality of life and social adaptation that the positive ones [71]. One patient with positive/negative symptoms was found to have average parameters of positive and negative SCZD symptoms. Diagnosis data are summarized in Table 1.
Table 1. General parameters for 35 patients
from three groups of SCZD patients
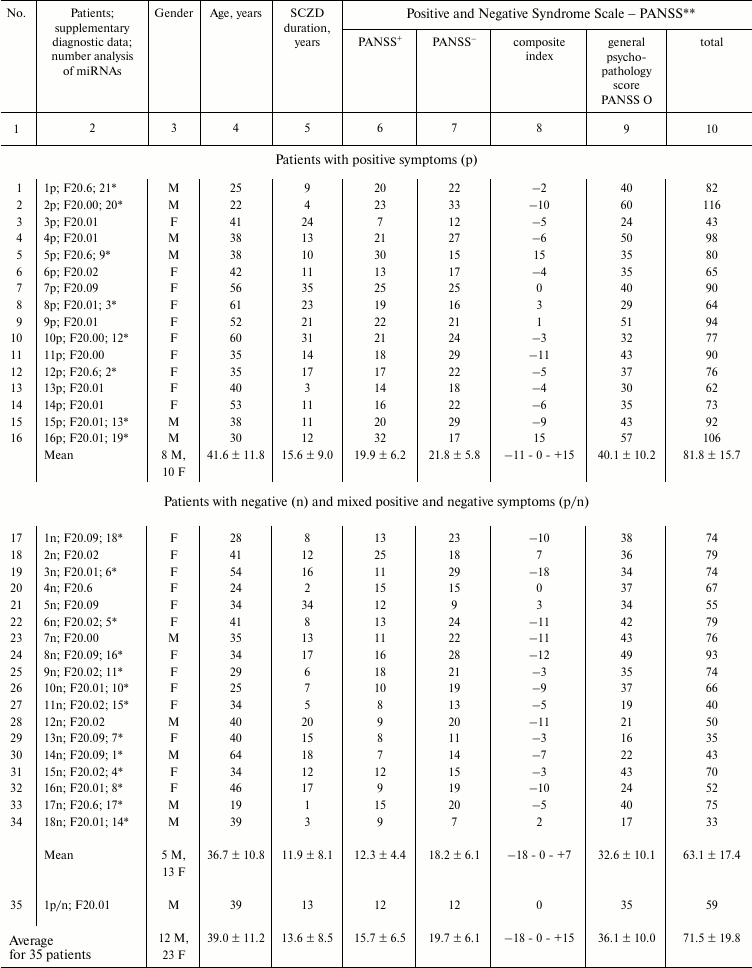
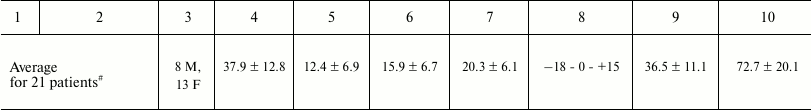
Note: Patients diagnosed with paranoid SCZD (ICD-10 code: F20.0; 30
patients) and simple SCZD (F20.6; 5 patients) were enrolled into the
study. Four patients were subtyped as F20.00 (paranoid), 13 as F20.01
(hebephrenic), 7 as F20.02 (catatonic), and 6 as F20.09 (unspecified).
PANSS scale allows standardized assessment of various psychopathology
symptoms in SCZD, to determine patient’s clinical profile, and to
follow up dynamic changes in the patient’s condition during
therapy. Each symptom is evaluated and presented as a score [48, 52].
PANSS+, scale for measuring positive (productive)
symptoms, such as delusions, mental disorders, hallucinations,
agitation, delusion of grandeur, suspicion, persecution mania, and
hostility. The symptoms are rated by comparing then to normal
psychological status. PANSS–, scale for
measuring negative symptoms, such as lowered emotional expression,
strangeness, communication difficulty, decrease initiative,
difficulties in abstract thinking, impaired spontaneous and fluid
speech, stereotyped thinking. The symptoms are rated by comparing then
to normal psychological status. Composite index is obtained by
subtracting the positive scale score from the negative scale score.
PANSS O, scale for general psychopathology syndromes, such as somatic
concerns (complaints of physical health problems), anxiety, feeling of
guilt, tension, mannerisms and posing, depression, motor retardation,
unsociable behavior, eccentric thoughts, disorientation, disturbance of
attention, disturbance of will, impulsivity, aggression, etc. General
SCZD severity is assessed. PANSS total, total score obtained by summing
up PANSS+, PANSS– and PANSS O.
* IgG preparations were tested for microRNA-hydrolyzing activity; the
assigned numbers are shown in the electrophoresis data (Figs. 2-5).
# Average for 21 patients marked with asterisk in the
table.
The patients were diagnosed with SCZD in accordance with ICD-10, and their diagnoses were clinically verified by the Department of Endogenous Disorders, Mental Health Research Institute (Tomsk, Russia). According to the provided information, all SCZD patients had a negative history of typical systemic autoimmune and rheumatic diseases.
This study was approved by the Mental Health Research Institute Ethics Committee, including patients’ written informed consent to use blood samples for scientific research, in accordance with the Declaration of Helsinki.
Antibody purification. Antibodies from the blood sera of 35 SCZD patients and 10 healthy donors were purified and analyzed by the earlier developed procedure [72-74] for purification of electrophoretically and immunologically homogenous IgG preparations from the human blood serum. The procedure included affinity chromatography of serum proteins on Protein G-Sepharose followed by high-performance gel-filtration on a Superdex-200 HR 10/30 column.
Antibody RNase activity assay. RNase activity of IgG preparations from SCZD patients was analyzed as described earlier [75, 76]. The reaction mixture (0.35 ml) contained 50 mM HEPES, pH 7.5 (or 50 mM Tris-HCl, pH 7.5, for poly(C) and microRNA), 0.05-0.20 mg/ml IgG, and one of the substrates: 0.2 mg/ml poly(C), 0.2 mg/ml poly(A), 50 µg/ml total yeast RNA, 1 mM cCMP, or 0.01 mg/ml microRNA (one of four). The hydrolysis rate was assessed from the changes in the absorbance at 250 nm (ΔE250 = 2380 M–1·cm–1) for poly(C) [77], poly(A), and total yeast RNA (ΔE282 = 829 M–1·cm–1 [78] or 292 nm for cCMP (ΔE292 = 730 M–1·cm–1) [79].
5′-Tagged miR-137 (5′-Flu-UUAUUGCUUAAGAAUACGCGUAG-3′), miR-9-5p (5′-Flu-UCUUUGGUUAUCUAGCUGUAUGA-3′), miR-219-2-3p (5′-Flu-AGAAUUGUGGCUGGACAUCUGU-3′), and miR-219a-5p (5′-Flu-UGAUUGUCCAAACGCAAUUCU-3′) contained fluorescein (Flu) at the 5′-end. Hydrolysis products were analyzed by electrophoresis in denaturing 20% polyacrylamide gel (0.1 M Tris, 0.1 M boric acid, 8 M urea, 0.02 M Na2EDTA, pH 8.3). The reaction mixture containing 0.1 mg/ml IgG was incubated for 1 h. The gels were analyzed using a Typhoon FLA 9500 laser scanner (GE Healthcare, USA). The volume of blood samples and the amount of purified IgG antibodies varied widely between the patients; therefore, experiments on microRNA hydrolysis were performed only with 21 out of 35 IgG preparations. An equimolar mixture of IgG preparations purified from the blood sera of 10 healthy donors (health-IgGmix) was used as a control. Special care was taken to select volunteers who were fully free of previous autoimmune, rheumatic, or allergic diseases, as well as viral and bacterial infections, at least within 2-3 years prior to the study.
Apparent Km and Vmax (kcat) values were calculated based on the reaction rate (V) dependence on the microRNA concentration using the Microcal Origin v.5.0 software and presented as the double-reciprocal Lineweaver–Burk plot [80]. The data obtained were shown as a mean ± standard deviation based on three independent experiments. The error of the estimations was less than 5-15%.
In situ analysis of the RNase activity for the equimolar mixture (10 µg) of 35 IgG preparations from SCZD patients (scz-IgGmix) was carried out in a denaturing 4-18% gradient polyacrylamide gel (0.1% SDS) containing 40 µg/ml yeast RNA as described earlier [75, 76].
MicroRNA 3D-modelling. MicroRNA 3D-models with the minimum energy were calculated with the Predict Secondary Structure software (http://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html).
Statistical analysis. The data were checked for the normal distribution using the Shapiro–Wilk W test. The majority of the parameters used for comparison did not fit the Gaussian distribution. For this reason, we used the non-parametric Spearman’s rank correlation coefficient test. In the case when the data fit the Gaussian distribution, we used the parametric Pearson’s correlation coefficient test was used. Differences between the samples were evaluated using the Mann–Whitney U test. Significance was set at p < 0.05. Median (M) and interquartile range (IQR) were determined.
RESULTS
Patients. Thirty-five SCZD patients with different disease symptoms according to the standard PANSS criteria [67-71] (Table 1) were divided into three groups: 16 – with positive symptoms (patients 1p-16p), 18 – with negative symptoms (patients 1n-18n), and 1 – with intermediate positive-negative symptoms (patient 1 p/n). Major PANSS parameters characterizing each of the groups are shown in Table 1.
Antibody purification. To examine the affinity and catalytic activity of abzymes in the blood serum from 35 SCZD patients, electrophoretically and immunologically homogeneous IgG preparations were obtained as described earlier [63-65, 72-75]. It was found that after gel-filtration in acidic buffer (pH 2.6), the maximal RNase activity coincided with the peak corresponding to scz-IgGmix. Immobilized mouse IgG antibodies raised against human IgG light chain fully absorbed this RNase activity.
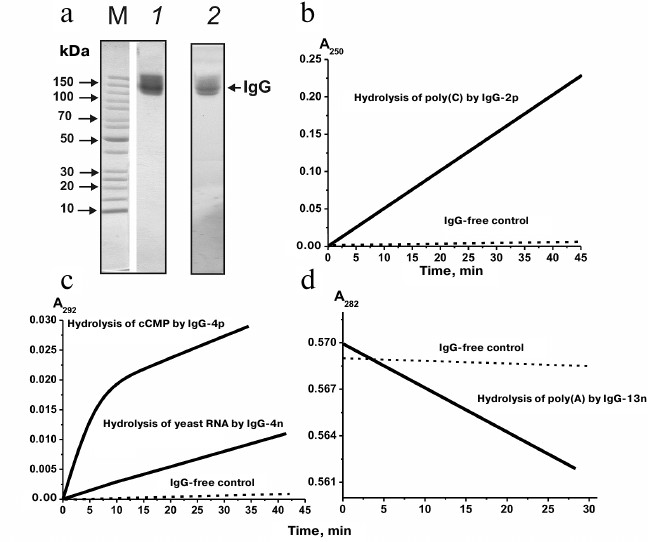
Figure 1a shows the results of in situ analysis of the RNase activity of IgG preparations after SDS-PAGE in a gradient 4-18% gel containing yeast RNA. Hydrolyzed RNA (seen as dark spots against fluorescent RNA background after gel staining with ethidium bromide) was observed only for the scz-IgGmix preparations. The fact that areas of RNA hydrolysis coincided with zones corresponding to intact scz-IgGmix, together with the lack of other areas of RNase activity (Fig. 1a), proved that IgG preparations were free from contamination with canonic RNases.
Fig. 1. a) Assay of RNase activity of IgGmix (10 µg; equimolar mixture of IgG antibodies from the blood sera of 35 SCZD patients) (lane 2) after electrophoresis in a gradient 4-18% SDS-polyacrylamide gel copolymerized with yeast RNA. RNase activity was measured from the emerging dark band against fluorescent background after gel staining with ethidium bromide. Protein positions were determined by staining with Coomassie R250 (lane 1). Lane M, protein molecular weight markers. b-d) Representative curves for poly(C), cCMP, yeast RNA, and poly(A) hydrolysis by IgG preparations (0.2 mg/ml) from different SCZD patients.
It has been shown before that IgG antibodies from the blood of healthy donors do not possess RNase activity [63-66]. Figure 1 (b-d) shows typical experimental curves for hydrolysis of poly(C), cCMP, yeast RNA, and poly(A). Similar dependences were obtained for all the examined substrates for all the 35 IgG preparations studied. The activities of these preparations varied among the patients; however, all 35 preparations demonstrated detectable or high levels of RNase activity (M/h per mg IgG) that declined in the order: cCMP (range, 0.3-1.7; median/IQR, 1.06/0.29); poly(C) (0.1-1.2; 0.36/0.2); poly(A) (0.09-0.50; 0.26/0.12); yeast RNA (0.04-0.48; 0.19/0.11).
The correlation coefficient (CC) for the parameters characterizing hydrolysis of the examined substrates for the entire group of patients was low except the CC value for cCMP and poly(C) hydrolysis (CC = –0.599) (Table 2). Interestingly, no clear correlation was observed between the RNase activity of 35 IgG preparations and duration of the disease for any of the four substrates (CC varied from –0.015 to +0.14). However, the level of cCMP hydrolysis correlated with the patient age (CC = +0.369; Table 2). Moreover, the RNase activity toward cCMP negatively correlated with the total PANSS score (CC = –0.45).
Table 2. Correlation between general
clinical parameters and enzymatic activity of antibodies from 35 SCZD
patients
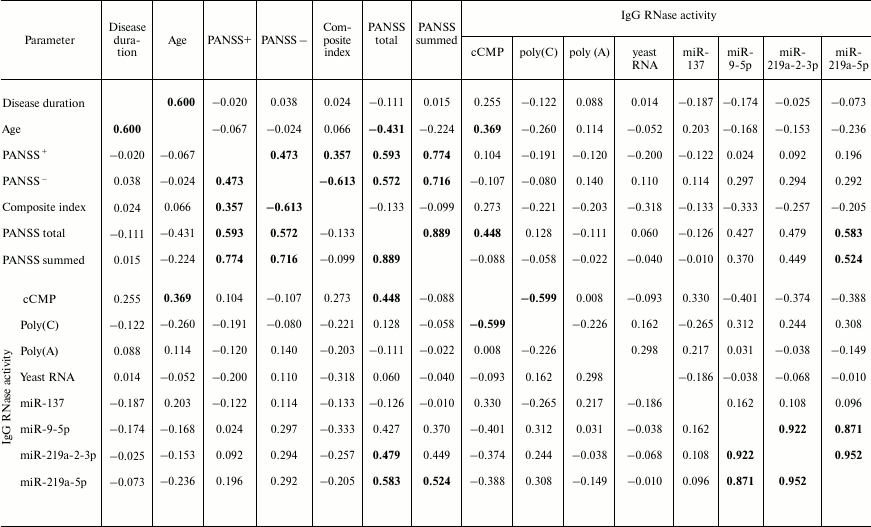
Note: Non-parametric Spearman’s rank correlation coefficient test was used except in cases of Gaussian distribution, when parametric Pearson’s correlation coefficient test was used (marked with *). The highest correlation coefficient values are shown in bold.
Thirty-five patients were divided into the three groups (Table 1). Mean RNase activities for IgG preparations from patients with positive and negative symptoms differed insignificantly: 1.01-fold for cCMP, 1.01-fold for poly(C), 1.08-fold for poly(A), and 1.14-fold for yeast RNA. No significant difference was found between the compared samples (Mann–Whitney test, p > 0.2).
For the entire group and patients with positive symptoms, the CC values for the parameters of cCMP, poly(C), poly(A), and yeast RNA hydrolysis were relatively low, either negative (from –0.056 to –0.185) or positive (from +0.135 to +0.394). Significant correlation was only found between the relative activities of cCMP and poly(C) hydrolysis (CC = –0.791). Also, positive correlation was observed between the level of cCMP hydrolysis and duration of the disease, as well as patient age (CC = +0.540 and +0.753, respectively). At the same time, the level of poly(C) hydrolysis negatively correlated with the patient age (CC = –0.748), i.e., the RNase activity decreased with age.
Similarly, the CC values for the IgG preparations from patients with negative symptoms were rather moderate, either negative (from –0.118 to –0.314) or positive (from +0.118 to +0.24). Analysis of the RNase activity dependence on clinical parameters showed that the level of yeast RNA hydrolysis correlated with the negative symptom scale score (CC = +0.535) and composite PANSS index (CC = –0.501).
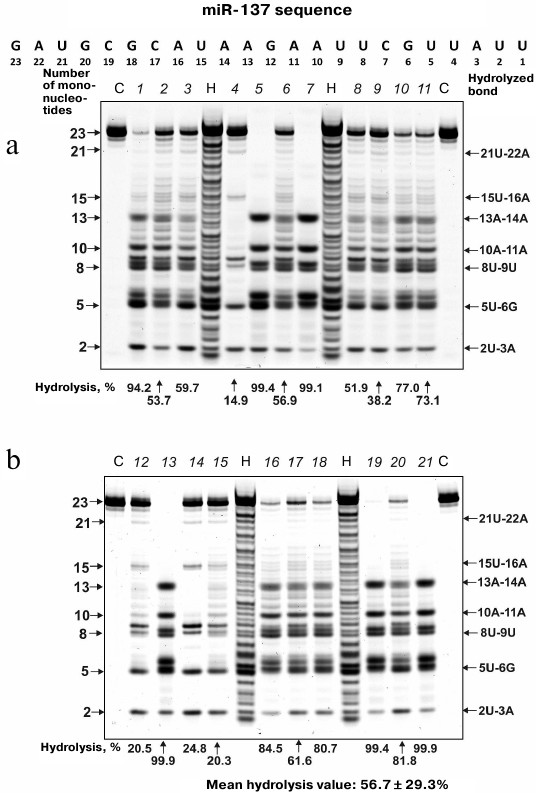
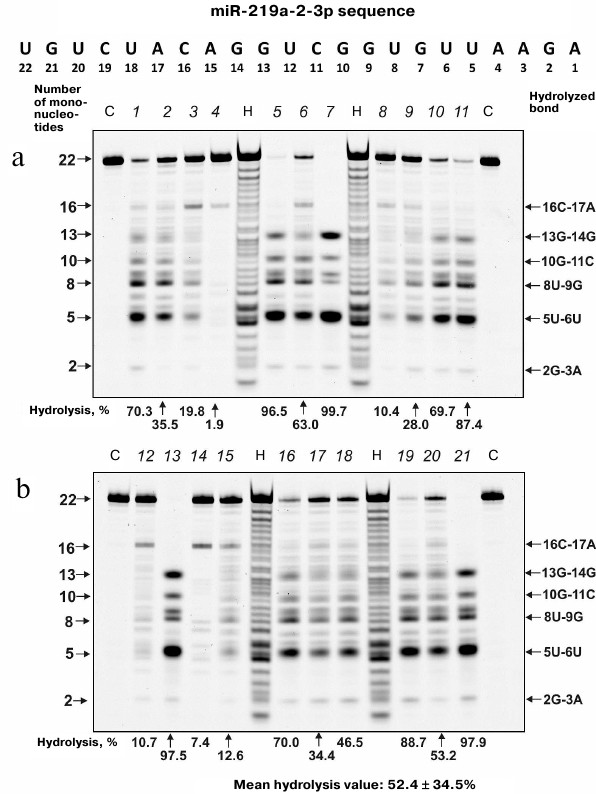
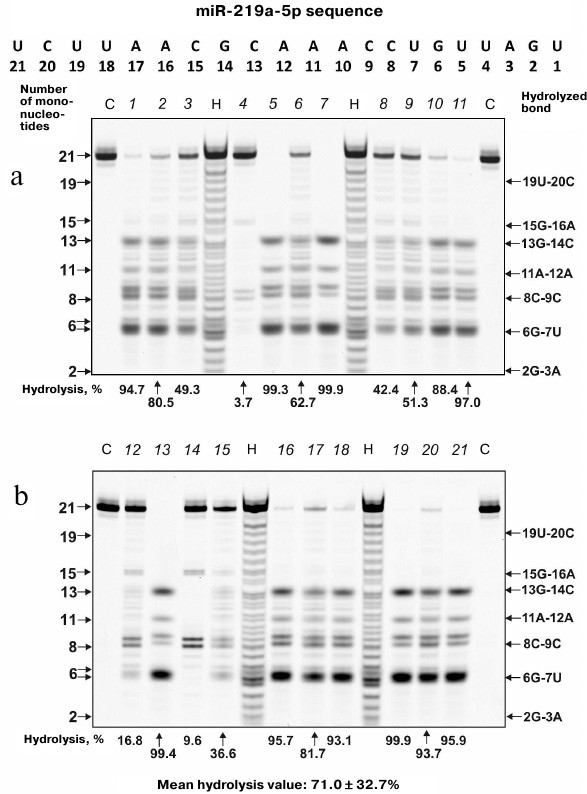
MicroRNA hydrolysis. As mentioned above, some microRNAs regulate up to several hundreds of genes in SCZD pathogenesis. Typical hydrolysis patterns for miR-137, miR-9-5p, miR-219-2-3p and miR-219a-5p including percentage of hydrolysis by each IgG preparation and mean values for 21 IgG preparations are shown in Figs. 2-5 (for the correspondence between the numbers of samples in the electrophoresis gels (1-21) and numbers assigned to patients with positive and negative symptoms see Table 1). We found that health-IgGmix displayed no activity toward the tested microRNAs.
Fig. 2. Hydrolysis patterns for Flu-miR-137 (0.01 mg/ml) cleaved with IgG preparations (0.1 mg/ml) from the blood sera of 21 SCZD patients. Hydrolysis products were detected from their fluorescence. Panels (a) and (b) show antibody with number assigned, product length, and percentage of site-specific RNA hydrolysis by each IgG preparation. C, Flu-miR-137 incubation in the absence of antibodies; H, statistical hydrolyzate of Flu-miR-137 (oligonucleotide length markers).
Fig. 3. Hydrolysis patterns for Flu-miR-219a-2-3p (0.01 mg/ml) cleaved with IgG preparations (0.1 mg/ml) from the blood sera of 21 SCZD patients. Hydrolysis products were detected from their fluorescence. Panels (a) and (b) show antibody with number assigned, product length, and percentage of site-specific RNA hydrolysis by each IgG preparation. C, Flu-miR-219a-2-3p incubation in the absence of antibodies; H, statistical hydrolyzate of Flu-miR-219a-2-3p (oligonucleotide length markers).
Fig. 4. Hydrolysis patterns for Flu-miR-219a-5p (0.01 mg/ml) cleaved with IgG preparations (0.1 mg/ml) from the blood sera of 21 SCZD patients. Hydrolysis products were detected from their fluorescence. Panels (a) and (b) show antibody with number assigned, product length, and percentage of site-specific RNA hydrolysis by each IgG preparation. C, Flu-miR-219a-5p incubation in the absence of antibodies; H, statistical hydrolyzate of Flu-miR-219a-5p (oligonucleotide length markers).
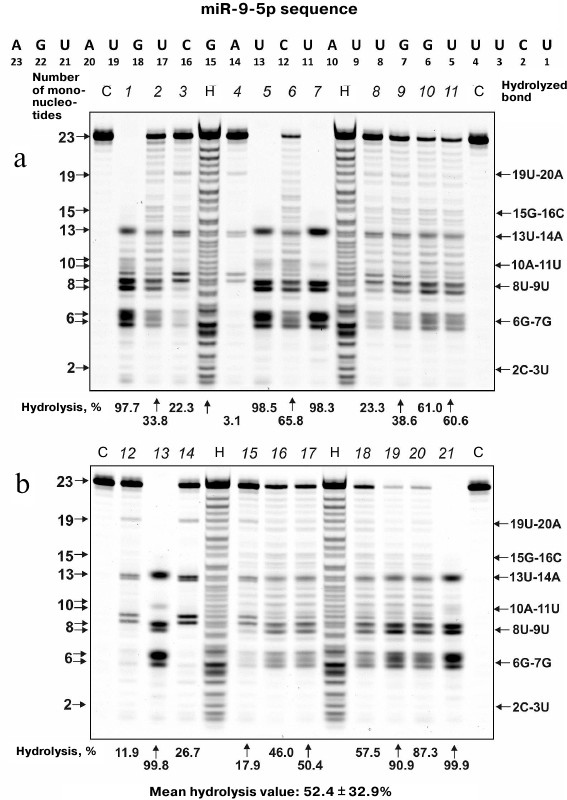
Fig. 5. Hydrolysis patterns for Flu-miR-9-5p (0.01 mg/ml) cleaved with IgG preparations (0.1 mg/ml) from the blood sera of 21 SCZD patients. Hydrolysis products were detected from their fluorescence. Panels (a) and (b) show antibody with number assigned, product length, and percentage of site-specific RNA hydrolysis by each IgG preparation. C, Flu-miR-9-5p incubation in the absence of antibodies; H, statistical hydrolyzate of Flu-miR-9-5p (oligonucleotide length markers).
The percentage of microRNA hydrolyzed by different IgG preparations under similar conditions differed; the mean values declined in the following order: miR-219a-5p (range, 7.4-99.7%; median/IQR, 88.4/46.6%) ≥ miR-137 (14.9-99.9%; 73.1/42.2%) ≥ miR-9-5p (3.1-99.9%; 57.5/64.2%) ≥ miR-219a-2-3p (7.4-99.7%; 53.2/67.7%) (Figs. 2-5). The correlation coefficients for the sets of relative activities for three examined miRNAs (miR-9-5p, miR-219a-2-3p and miR-219a-5p) were rather high (from +0.87 to +0.95). The CC values for the correlation between the relative activities toward miR-137 and three other miRNAs were relatively low and varied from +0.096 to +0.162 (Table 2).
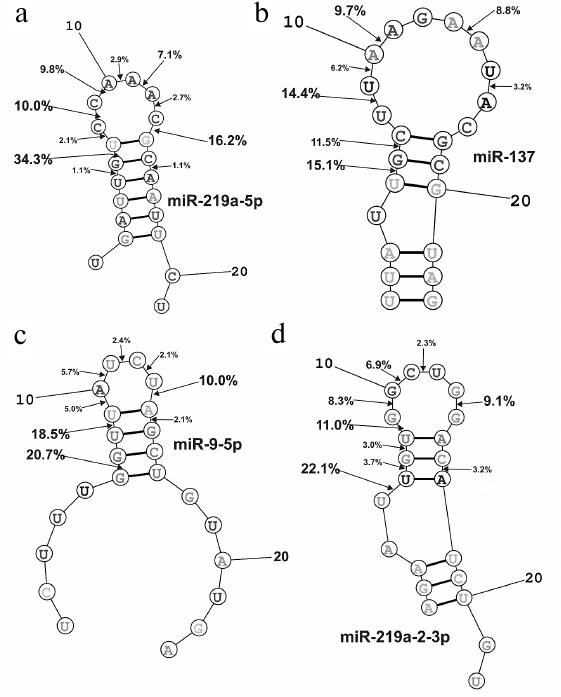
It is known that in solution microRNAs adopt a hairpin structure. We calculated the 3D minimal-energy hairpin structures for all the microRNAs studied. The sites for the most active and moderate hydrolysis in these microRNAs are shown in Fig. 6, as well as average percentage of their hydrolysis by 21 IgG preparations. It is evident that the three major cleavage sites for all microRNAs are located in the loops or adjacent duplex regions. These sites differ among the four microRNAs; however, in all of them, RNA cleavage occurs either after or before G, although other possibilities exist: 6G-7U, 13C-14G, and 8C-9C in miR-219a-5p; 5U-6G, 8U-9U, and 10A-11A in miR-137; 6G-7G, 8U-9U, and 13U-14A in miR-9-5p; 5U-6U, 8U-9G, and 13G-14G in miR-219a-2-3p (Fig. 6).
Fig. 6. Calculated 3D-structures for four microRNAs: miR-219a-5p (a), miR-137 (b), miR-9-5p (c), and miR-219a-2-3p (d). The average efficiency of microRNA hydrolysis at major and moderate cleavage sites by 21 IgG preparations is shown.
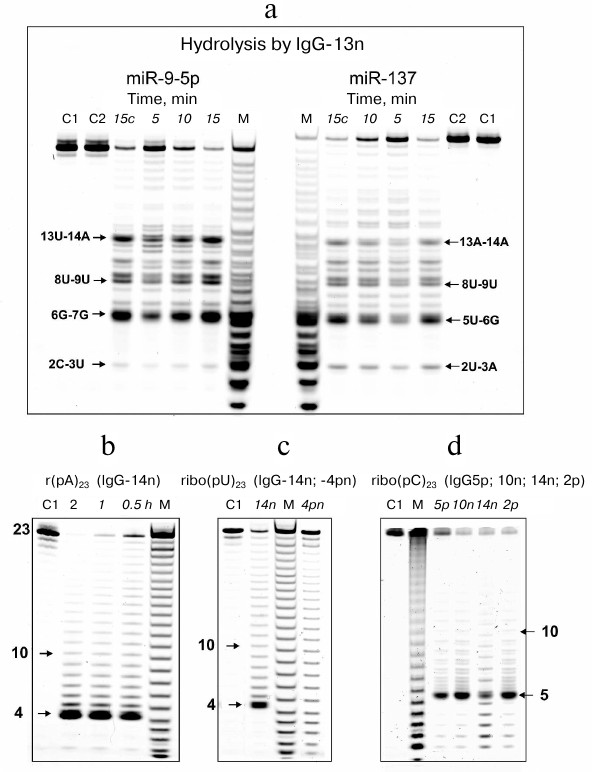
Next, the microRNAs were denatured by heating up to 75°C followed by quick cooling on ice. Representative hydrolysis patterns for miR-9-5p and miR-137 by IgG-13n before and after denaturing clearly show that the major hydrolysis sites in these microRNAs were preserved after denaturation, although the relative amounts of hydrolysis products slightly differed (Fig. 7a). The most important difference was increased amounts of fragments 14 to 22 nucleotides in length in the hydrolysis products of the denatured microRNAs. Similar patterns were observed for the cleavage of miR-219-5p and miR-219a-2-3p by IgG-13n and for all four microRNAs hydrolyzed by other IgG preparations. Therefore, the cleavage of microRNA at the major sites depends on the microRNA sequence rather than its unique structure.
Fig. 7. a) Hydrolysis of Flu-miR-9-5p and Flu-miR-137 (0.01 mg/ml) by IgG-13n (0.2 mg/ml) before and after miRNA denaturation. Hydrolysis products were detected from their fluorescence after reaction mixture incubation for 5-15 min; lane 15c corresponds to microRNA before denaturation. b-d) Hydrolysis patterns for Flu-r(pA)23 (b), Flu-r(pU)23 (c), and Flu-r(pC)23 (d) cleaved with IgG preparations from different SCZD patients at various incubation times. Numbers assigned to IgG preparation are shown. Lanes C1 and C2 correspond to nondenatured and denatured microRNAs, respectively, incubated in the absence of antibodies; lane M, oligonucleotide length markers.
It seemed interesting to identify the sites of abzyme-catalyzed cleavage in homo-oligonucleotides of similar length. Typical patterns for r(pA)23, r(pU)23 and r(pC)23 hydrolysis by some of the IgG preparations are demonstrated in Fig. 7, b-d. In contrast to microRNAs, hydrolysis of homo-oligonucleotides was less specific and occurred evenly along the entire length of the homo-oligonucleotide, except more pronounced cleavage between the 5´-terminal A4 and A5 nucleotides in r(pA)23, U4 and U5 in r(pU)23, and C5 and C6 in r(pC)23. These were the only major sites detected for all the examined IgG preparations.
We also found significant correlation between the microRNA-hydrolyzing activity and clinical parameters (according to Spearman’s correlation coefficient test). Thus, the levels of miR-219a-2-3p and miR-219a-5p hydrolysis by IgG preparations from SCZD patients positively correlated with the PANSS general symptom score (CC = +0.479 and +0.583, respectively). The level of miR-219a-5p hydrolysis positively correlated with the PANSS total score (CC = +0.524).
Analysis of correlation in the group of patients with positive SCZD symptoms revealed that the level of miR-9-5p hydrolysis displayed a strong negative correlation with the duration of the disease (CC = –0.783), i.e., the microRNA-hydrolyzing activity declined with age. A noticeable positive correlation was observed between the RNase activity against miR-9-5p, miR-219-2-3p, and miR-219-5p and PANSS general symptom score, as well as total PANSS score (CC > 0.75).
No significant correlation was found for the patients with negative symptoms.
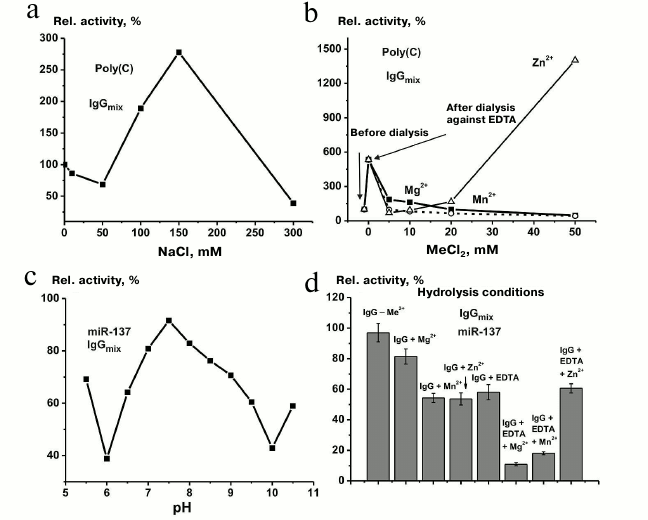
Effect of reaction conditions on RNA hydrolysis. The optimal ionic strength for polynucleotide hydrolysis by scz-IgGmix was determined for poly(C): the maximum hydrolysis was observed at 150 mM NaCl, i.e., under physiological conditions (Fig. 8a). After IgGmix was dialyzed against EDTA, its relative activity increased ~5-fold (Fig. 8b). Further addition of MgCl2 and MnCl2 resulted in the reaction inhibition, while addition of Zn2+ ions to the dialyzed IgGmix strongly activated RNA hydrolysis (Fig. 8b). It should be noted that classic RNases are site-specific (e.g., RNase T1 selectively cleaves RNA to the right of G bases, RNase A – to the right of pyrimidines) [64-66]. Nuclease S1 selectively cleaves only single-stranded RNA fragments. The abzymes from SCZD patients displayed none of these types of specificity.
Fig. 8. The dependence of the efficiency of IgGmix-catalyzed hydrolysis of poly(C) on the ionic strength (a) and metal ion concentration (b). The dependence of the efficiency of IgGmix-catalyzed hydrolysis of miR-137 on the reaction mixture pH (c) and the presence of 10 mM EDTA and 40 mM metal ions (d).
The activity of classic human RNases does not depend on the presence of metal ions [81]. At the same time, the activity of IgG antibodies derived from the blood serum of AID patients may either depend on metal ions or be unaffected by them [64-66]. Previously, it was shown that the purification procedure used in our study results in antibody preparations containing small amounts of metal ions bound to the antibodies [82]. The maximum miR-137 hydrolysis by scz-IgGmix was observed in the absence of any added components. The presence of 10 mM EDTA in the reaction mixture resulted in approximately 1.7-fold decrease in the RNase activity. Mn2+ and Zn2+ ions reduced the activity ~1.8-fold, whereas Mg2+ reduced it only 1.2-fold (Fig. 8). It is interesting that simultaneous presence of EDTA and Mg2+ or Mn2+ ions in the reaction mixture inhibited the RNase activity more profoundly (5.2-9.3 times) than each of these agents separately. The combination of EDTA and Zn2+ ions reduced the activity only 1.6-fold.
Abzymes from SLE and MS patients were found to display novel RNase activity stimulated by Mg2+ [64-66]. An example of such activity is hydrolysis of human mitochondrial tRNALys and its A50G mutant version by abzymes from SLE patients [64-66]. When these tRNAs were hydrolyzed with RNase A and other RNases traditionally used to analyze structural variations, no differences in the cleavage patterns were observed [64-66]. However, when the same molecules were cleaved with the abzymes in the presence of Mg2+ ions, different hydrolysis products were obtained, which indicated formation of new cleavage sites in the mutant molecule and suggested local structural or conformational changes in the mutant molecule [64-66]. In contrast to the abzymes from SLE and MS patients, Mg2+ ions inhibited rather than activated RNA hydrolysis by antibodies from SCZD patients (Fig. 8, b and d). After dialysis, abzymes from SCZD patients displayed the maximum activity in the presence of Zn2+ ions (Fig. 8, b and d). Therefore, it cannot be ruled out that a small fraction of abzymes from SCZD patients are Zn2+-dependent RNases.
The pH optimum for miR-137 hydrolysis by scz-IgGmix was close to pH 7.5 (Fig. 8c).
We also determined apparent Km and Vmax (kcat) values for microRNA hydrolysis by some IgG preparations. The relationship between the initial hydrolysis rate and microRNA concentration in the IgG-catalyzed reaction corresponded to the Michaelis–Menten kinetics. The Km and kcat values were comparable for all the examined microRNAs: Km = 3.5 ± 0.2 µM and kcat = 0.14 ± 0.009 min–1 for miR-137; Km = 2.4 ± 0.13 µM and kcat = 0.083 ± 0.003 min–1 for miR-9-5p; Km = 1.7 ± 0.12 µM and kcat = 0.10 ± 0.008 min–1 for miR-219-2-3p; Km = 4.5 ± 0.2 µM and kcat = 0.17 ± 0.02 min–1 for miR-219a-5p.
DISCUSSION
Previously, we have demonstrated that antibodies from healthy donors do not hydrolyze RNA and DNA [64-66]. However, numerous published studies have demonstrated the manifestations of autoimmune responses in SCZD patients. SCZD was found to be associated with AIDs in many patients (see above; [8, 13, 22-36]). However, SCZD is not yet considered a typical AID. At the same time, IgG antibodies with the DNase, RNase, proteolytic, or amylase activities are the earliest significant markers of AIDs [59, 60]. Antibodies from SCZD patients efficiently hydrolyze DNA and MBP [61, 62].
Here, we demonstrated that IgG antibodies from SCZD patients have intrinsic RNase activity. While IgG antibodies from 90-95% SLE and MS patients efficiently hydrolyze DNA and MBP [55-58], such activity was documented only for IgG antibodies from 80-82% SCZD patients [61, 62]. However, we found that all examined IgG preparations (100%) from SCZD patients efficiently hydrolyzed cCMP, various homo-oligonucleotides, and microRNAs. Except for cCMP and poly(C) (CC = –0.599), no significant correlation was observed for the IgG-catalyzed hydrolysis of various substrates (Table 2); although correlation with some SCZD clinical parameters was found.
Earlier, we were able to generate RNA-hydrolyzing abzymes by vaccination of rabbits with DNA, RNA, DNase I, DNase II, and pancreatic RNase [60, 75]. Hence, it is possible that the blood serum of patients with different pathologies contains abzymes specific toward different RNAs. We demonstrated that all four microRNAs examined were hydrolyzed in a site-specific manner (Figs. 2-5). However, the fact that the major hydrolysis products for all homo-r(pN)23 were 4- to 6-nucleotide-long oligonucleotides (Fig. 7, b-d) deserves special attention. All four examined microRNAs were also cleaved into short fragments of similar length (Figs. 2-5, a-d). It has been shown before that the abzyme active site is localized in its light chain that strongly binds only to 4-5 nucleotides in an oligonucleotide [83]. The contribution of other nucleotides to the binding decreases, and the dependence of the binding affinity on the oligonucleotide length reaches a plateau at n ≥ 8-9 regardless of the total oligonucleotide length. Therefore, it might be assumed that the antibody light chains efficiently recognize 4-6 5′-terminal nucleotides in any RNA. This might be the reason why abzymes hydrolyze RNA mostly with the formation of fragments 4-6 nucleotides in length. At the same time, the existence of longer, less efficiently generated products, suggests that the light chains could form complexes with regions that are positioned away from the 5′-termini in oligonucleotides and microRNAs. The presence of minor, but nevertheless significant, sites of hydrolysis in microRNAs (Figs. 2-5, a-d) suggests that specific RNAs could bind to antibodies via alternative mechanisms. Moreover, it cannot be ruled out that generation of 4- to 6-nucleotide-long oligonucleotides is the result of binding and subsequent hydrolysis of any RNA substrates by either sequence-specific or non-specific abzymes. Most likely, generation of major microRNA hydrolysis products is due to their cleavage by antibodies specific toward these RNAs.
Usually, antibody-dependent catalysis is characterized with 102-106 times lower kcat values as compared to canonical enzymes (see [54-58] and in-text citations). This higher substrate affinity of abzymes results in lower kcat values, since increase in the affinity leads to prolonged lifetime of the antibody–substrate complex, and, as a consequence, lower catalyst turnover number. For abzymes from AID patients, kcat values vary within the 10–6-40 min–1 range (see [54-58] and in-text citations).
The apparent kcat values for the abzyme-mediated microRNA hydrolysis were relatively high (0.083-0.17 min–1), although still lower by approximately one order of magnitude than those observed for the blood serum antibodies from SLE patients [76, 84]. Because the specific activity of the antibodies was calculated based on the total IgG concentration, specific RNase activities of individual monoclonal subfractions could be substantially higher than that of intact polyclonal IgGs.
The percentage of SCZD patients with high or reliably identified RNase activity (100%) is substantially higher than that of SCZD patients with the DNase activity (~80%) [61]. This might be related to the fact that the RNase activity of an antibody preparation is usually 30-300 times higher than its DNase activity, depending on a patient and disease course [39-42, 44, 47, 56, 57, 63, 68], so that the RNase activity may be detected in preparations with the DNase activity that is too low to be identified. It cannot be excluded that virtually all SCZD patients, whose clinical parameters are close to those shown in Table 1, might have in their blood serum antibodies displaying RNase activity.
Here, we demonstrated for the first time that polyclonal IgG antibodies purified from the blood serum of SCZD patients possess RNase activity. The presence of abzymes with the RNase, DNase, and proteolytic activities suggest the development of autoimmune processes in SCZD patients. MicroRNA-hydrolyzing abzymes might interfere with the transcription of the corresponding genes and biosynthesis of their protein products.
Recent studies performed at London Medical Institute Oliver House support the theory that SCZD is a consequence of autoimmune brain disorder [85]. As mentioned above, abzymes are the earliest markers of the developing autoimmune response. In mice, the pre-disease stages of spontaneous and antigen-triggered SLE and EAE (model of experimental allergic encephalomyelitis or MS) are associated with the changes in the differentiation profile of bone marrow stem cells. These changes become even more pronounced with further development of the pathological process [59, 86-89] and are related to the emergence of abzymes hydrolyzing DNA, MBP, ATP, and polysaccharides in the mouse blood serum. The activity toward DNA, MBP, and polysaccharides of antibodies in the cerebrospinal fluid of MS patients was 30-60 times higher (depending on the substrate type) than in the blood of the same patients [90-92]. It is possible that the autoimmune disease development starts in the cerebrospinal fluid and the brain, which is in a good agreement with the theory [85] that SCZD results from the impairments in the brain immune system.
Acknowledgments
We are thankful to A. V. Semke and N. A. Bokhan for kindly providing blood samples collected from schizophrenia patients and to A. G. Venyaminova and M. A. Vorobyeva for synthesizing specific oligonucleotide probes and assisting in performing experiments.
This study was supported by the Russian Science Foundation (project 16-15-10103). Collection of blood serum samples from schizophrenia patients and purification with partial characterization of antibodies were supported by the SubProgram 1 of the Integrated Program of the Siberian Branch of the Russian Academy of Sciences (III.2P.1/VI.57-5, 0309-2015-0022) and the Russian Foundation for Basic Research (project 16-04-00603).
REFERENCES
1.Meyer-Lindenberg, A. (2011) Neuroimaging and the
question of neurodegeneration in schizophrenia, Prog.
Neurobiol., 95, 514-516.
2.Goldner, E. M., Hsu, L., Waraich, P., and Somers,
J. M. (2002) Prevalence and incidence studies of schizophrenic
disorders: a systematic review of the literature, Can. J.
Psychiatry, 47, 833-843.
3.Beasley, C. L., Pennington, K., Behan, A., Wait,
R., Dunn, M. J., and Cotter, D. (2006) Proteomic analysis of the
anterior cingulate cortex in the major psychiatric disorders: evidence
for disease-associated changes, Proteomics, 6,
3414-3425.
4.Lewis, D. A., Hashimoto, T., and Volk, D. W. (2005)
Cortical inhibitory neurons and schizophrenia, Nat. Rev.
Neurosci., 6, 312-324.
5.Reynolds, L. M., and Reynolds, G. P. (2011)
Differential regional N-acetylaspartate deficits in postmortem brain in
schizophrenia, bipolar disorder and major depressive disorder, J.
Psychiatr. Res., 45, 54-59.
6.Jenkins, T. A., Harte, M. K., Stenson, G., and
Reynolds, G. P. (2009) Neonatal lipopolysaccharide induces pathological
changes in parvalbumin immunoreactivity in the hippocampus of the rat,
Behav. Brain Res., 205, 355-359.
7.Mura, G., Petretto, D. R., Bhat, K. M., and Carta,
M. G. (2012) Schizophrenia: from epidemiology to rehabilitation,
Clin. Pract. Epidemiol. Ment. Health, 8, 52-66.
8.Strous, R. D., and Shoenfeld, Y. (2006)
Schizophrenia, autoimmunity and immune system dysregulation: a
comprehensive model updated and revisited, J. Autoimmun.,
27, 71-80.
9.Muller, N., Weidinger, E., Leitner, B., and
Schwarz, M. J. (2015) The role of inflammation in schizophrenia,
Front. Neurosci., 9, 372.
10.Muller, N., and Schwarz, M. (2006) Schizophrenia
as an inflammation-mediated disbalance of glutamatergic
neurotransmission, Neurotox. Res., 10, 131-148.
11.Muller, N., Riedel, M., Gruber, R., Ackenheil,
M., and Schwarz, M. J. (2000) The immune system and schizophrenia: an
integrative view, Ann. NY Acad. Sci., 917, 456-467.
12.Muller, N., and Schwarz, M. J. (2010) The role of
immune system in schizophrenia, Curr. Immunol. Rev., 6,
213-220.
13.Bergink, V., Gibney, S. M., and Drexhage, H. A.
(2014) Autoimmunity, inflammation, and psychosis: a search for
peripheral markers, Biol. Psychiatr., 75, 324-331.
14.Strous, R. D., and Shoenfeld, Y. (2006)
Schizophrenia, autoimmunity and immune system dysregulation: a
comprehensive model updated and revisited, J. Autoimmun.,
27, 71-80.
15.Jones, A. L., Mowry, B. J., Pender, M. P., and
Greer, J. M. (2005) Immune dysregulation and self-reactivity in
schizophrenia: do some cases of schizophrenia have an autoimmune basis?
Immunol. Cell Biol., 83, 9-17.
16.Sperner-Unterweger, B., and Fuchs, D. (2015)
Schizophrenia and psychoneuroimmunology: an integrative view, Curr.
Opin. Psychiatr., 28, 201-206.
17.Ripke, S., O’Dushlaine, C., Chambert, K.,
Moran, J. L., Kahler, A. K., Akterin, S., Bergen, S., Collins, A.L.,
Crowley, J.J., Fromer, M., Kim, Y., Lee, S.H., Magnusson, P.K.E.,
Sanchez, N., Stahl, E.A., Williams, S., Wray, N.R., Xia, K., Bettella,
F., Borglum, A.D., Bulik-Sullivan, B.K., Cormican, P., Craddock, N., de
Leeuw, C., Durmishi, N., Gill, M., Golimbet, V., Hamshere, M.L.,
Holmans, P., Hougaard, D.M., Kendler, K.S., Lin, K., Morris, D.W.,
Mors, O., Mortensen, P.B., Neale, B.M., O'Neill, F.A., Owen, M.J.,
Milovancevic, M.P., Posthuma, D., Powell, J., Richards, A.L., B.P.
Riley, Ruderfer, D., Rujescu, D., Sigurdsson, E., Silagadze, T., Smit,
A.B., Stefansson, H., Steinberg, S., Suvisaari, J., Tosato, S.,
Verhage, M., Walters, J.T., Multicenter Genetic Studies of
Schizophrenia Consortium, Psychosis Endophenotypes Consortium, Wellcome
Trust Case-Control Consortium, Bramon, E., Corvin, A.P., O'Donovan,
M.C., Stefansson, K., Scolnick, E., Purcell, S., McCarroll, S., Sklar,
P., Hultman, C.M., and Sullivan P.F. (2013) Genome-wide association
analysis identifies 13 new risk loci for schizophrenia, Nat.
Genet., 45, 1150-1159.
18.Schizophrenia Psychiatric Genome-Wide Association
Study (GWAS) Consortium (2011) Genome-wide association study identifies
five new schizophrenia loci, Nat. Genet., 43,
969-976.
19.Sekar, A., Bialas, A. R., de Rivera, H., Davis,
A., Hammond, T. R., Kamitaki, N., Tooley, K., Presumey, J., Baum, M.,
Van Doren, V., Genovese, G., Rose, S. A., Handsaker, R. E.,
Schizophrenia Working Group of the Psychiatric Genomics Consortium,
Daly, M. J., Carroll, M. C., Stevens, B., and McCarroll, S. A. (2016)
Schizophrenia risk from complex variation of complement component 4,
Nature, 530, 177-183.
20.Potvin, S., Stip, E., Sepehry, A. A., Gendron,
A., Bah, R., and Kouassi, E. (2008) Inflammatory cytokine alterations
in schizophrenia: a systematic quantitative review, Biol.
Psychiatr., 63, 801-808.
21.McKernan, D. P., Dennison, U., Gaszner, G.,
Cryan, J. F., and Dinan, T. G. (2011) Enhanced peripheral toll-like
receptor responses in psychosis: further evidence of a pro-inflammatory
phenotype, Transl. Psychiatr., 1, e36.
22.Pollak, T. A., Beck, K., Irani, S. R., Howes, O.
D., David, A. S., and McGuire, P. K. (2016) Autoantibodies to central
nervous system neuronal surface antigens: psychiatric symptoms and
psychopharmacological implications, Psychopharmacology,
233, 1605-1621.
23.Klyushnik, T. P., Dupin, A. M., Siryachenko, T.
M., Sarmanova, Z. V., Otman, I. N., and Sokolov, R. E. (2008) Dynamics
of neuroantigen-specific antibodies in the blood serum from patients
with schizophrenia during therapy, Zh. Nevrol. Psikhiatr. im. S. S.
Korsakova, 108, 61-65.
24.Deakin, J., Lennox, B. R., and Zandi, M. S.
(2014) Antibodies to the N-methyl-D-aspartate receptor and other
synaptic proteins in psychosis, Biol. Psychiatr., 75,
284-291.
25.Steiner, J., Schiltz, K., Bernstein, H. G., and
Bogerts, B. (2015) Antineuronal antibodies against neurotransmitter
receptors and synaptic proteins in schizophrenia: current knowledge and
clinical implications, CNS Drugs, 29, 197-206.
26.Levite, M. J. (2014) Glutamate receptor
antibodies in neurological diseases, J. Neural. Transom.
(Vienna), 121, 1029-1075.
27.Eaton, W. W., Byrne, M., Ewald, H., Mors, O.,
Chen, C. Y., Agerbo, E., and Mortensen, P. B. (2006) Association of
schizophrenia and autoimmune diseases: linkage of Danish national
registers, Am. J. Psychiatry, 163, 521-528.
28.Tiosano, S., Farhi, A., Watad, A., Grysman, N.,
Stryjer, R., Amital, H., Comaneshter, D., Cohen, A. D., and Amital, D.
(2017) Schizophrenia among patients with systemic lupus erythematosus:
population-based cross-sectional study, Epidemol. Psychiatr.
Sci., 26, 424-429.
29.Mack, A., Pfeiffer, C., Schneider, E. M., and
Bechter, K. (2017) Schizophrenia or atypical lupus erythematosus with
predominant psychiatric manifestations over 25 years: case
analysis and review, Front. Psychiatry, 8, 131.
30.Arneth, B. M. (2017) Multiple sclerosis and
schizophrenia, Int. J. Mol. Sci., 12, 18.
31.Othman, S. S., Kadir, K. A., Hassan, J., Hong, G.
K., Singh, B. B., and Raman, N. (1994) High prevalence of thyroid
function test abnormalities in chronic schizophrenia, Aust. NZ J.
Psychiatr., 28, 620-624.
32.Hardoy, M. C., Cadeddu, M., Serra, A., Moro, M.
F., Mura, G., Mellino, G., Bhat, K. M., Altoe, G., Usai, P., Piga, M.,
and Carta, M. G. (2011) A pattern of cerebral perfusion anomalies
between major depressive disorder and Hashimoto thyroiditis, BMC
Psychiatry, 11, 148.
33.Endres, D., Dersch, R., Hochstuhl, B., Fiebich,
B., Hottenrott, T., Perlov, E., Maier, S., Berger, B., Baumgartner, A.,
Venhoff, N., Stich, O., and Tebartz van Elst, L. (2017) Intrathecal
thyroid autoantibody synthesis in a subgroup of patients with
schizophrenia form syndromes, J. Neuropsychiatry Clin.
Neurosci., 29, 365-374.
34.Haider, A. S., Alam, M., Adetutu, E., Thakur, R.,
Gottlich, C., DeBacker, D. L., and Marks, L. (2016) Autoimmune
schizophrenia? Psychiatric manifestations of Hashimoto’s
encephalitis, Cureus, 8, e672.
35.Kridin, K., Zelber-Sagi, S., Comaneshter, D., and
Cohen, A. D. (2017) Association between schizophrenia and an autoimmune
bullous skin disease-pemphigus: a population-based large-scale study,
Epidemiol. Psychiatr. Sci., 25, 1-8.
36.Regina, P., Pnina, R., Natur, F., and Yair, L.
(2017) Anti-phospholipid syndrome associated with schizophrenia
description of five patients and review of the literature, Immunol.
Res., 65, 438-446.
37.Miller, B. H., and Wahlestedt, C. (2010) MicroRNA
dysregulation in psychiatric disease, Brain Res., 1338,
89-99.
38.Sun, X. Y., Lu, J., Zhang, L., Song, H. T., Zhao,
L., Fan, H. M., Zhong, A. F., Niu, W., Guo, Z. M., Dai, Y. H., Chen,
C., Ding, Y. F., and Zhang, L. Y. (2015) Aberrant microRNA expression
in peripheral plasma and mononuclear cells as specific blood-based
biomarkers in schizophrenia patients, J. Clin. Neurosci.,
22, 570-574.
39.Shi, W., Du, J., Qi, Y., Liang, G., Wang, T., Li,
S., Xie, S., Zeshan, B., and Xiao, Z. (2012) Aberrant expression of
serum miRNAs in schizophrenia, J. Psychiat. Res., 46,
198-204.
40.Lai, C. Y., Yu, S. L., Hsieh, M. H., Chen, C. H.,
Chen, H. Y., Wen, C. C., Huang, Y. H., Hsiao, P. C., Hsiao, C. K., Liu,
C. M., Yang, P. C., Hwu, H. G., and Chen, W. J. (2011) MicroRNA
expression aberration as potential peripheral blood biomarkers for
schizophrenia, PloS One, 6, e21635.
41.Lai, C. Y., Lee, S. Y., Scarr, E., Yu, Y. H.,
Lin, Y. T., Liu, C. M., Hwang, T. J., Hsieh, M. H., Liu, C. C., Chien,
Y. L., Udawela, M., Gibbons, A. S., Everall, I. P., Hwu, H. G., Dean,
B., and Chen, W. J. (2016) Aberrant expression of microRNAs as
biomarker for schizophrenia: from acute state to partial remission, and
from peripheral blood to cortical tissue, Transl. Psychiat.,
6, e717.
42.Perkins, D. O., Jeffries, C. D., Jarskog, L. F.,
Thomson, J. M., Woods, K., Newman, M. A., Parker, J. S., Jin, J., and
Hammond, S. M. (2007) MicroRNA expression in the prefrontal cortex of
individuals with schizophrenia and schizoaffective disorder, Genome
Biol., 8, R27.
43.Moreau, M. P., Bruse, S. E., David-Rus, R.,
Buyske, S., and Brzustowicz, L. M. (2011) Altered microRNA expression
profiles in postmortem brain samples from individuals with
schizophrenia and bipolar disorder, Biol. Psychiat., 69,
188-193.
44.Hauberg, M. E., Roussos, P., Grove, J., Borglum,
A. D., and Mattheisen, M. (2016) Analyzing the role of microRNAs in
schizophrenia in the context of common genetic risk variants, JAMA
Psychiat., 73, 369-377.
45.Yin, J., Lin, J., Luo, X., Chen, Y., Li, Z., Ma,
G., and Li, K. (2014) miR-137: a new player in schizophrenia, Int.
J. Mol. Sci., 15, 3262-3271.
46.Olde Loohuis, N. F., Ba, W., Stoerchel, P. H.,
Kos, A., Jager, A., Schratt, G., Martens, G. J., van Bokhoven, H.,
Nadif Kasri, N., and Aschrafi, A. (2015) MicroRNA-137 controls
AMPA-receptor-mediated transmission and mGluR-dependent LTD, Cell
Rep., 11, 1876-1884.
47.Sakamoto, K., and Crowley, J. J. (2018) A
comprehensive review of the genetic and biological evidence supports a
role for microRNA-137 in the etiology of schizophrenia, Am. J.
Med. Genet. B Neuropsychiatr. Genet., 177, 242-256.
48.Siegert, S., Seo, J., Kwon, E. J., Rudenko, A.,
Cho, S., Wang, W., Flood, Z., Martorell, A. J., Ericsson, M.,
Mungenast, A. E., and Tsai, L. H. (2015) The schizophrenia risk gene
product miR-137 alters presynaptic plasticity, Nat. Neurosci.,
18, 1008-1016.
49.Topol, A., Zhu, S., Hartley, B. J., English, J.,
Hauberg, M. E., Tran, N., Rittenhouse, C. A., Simone, A., Ruderfer, D.
M., Johnson, J., Readhead, B., Hadas, Y., Gochman, P. A., Wang, Y. C.,
Shah, H., Cagney, G., Rapoport, J., Gage, F. H., Dudley, J. T., Sklar,
P., Mattheisen, M., Cotter, D., Fang, G., and Brennand, K. J. (2016)
Dysregulation of miRNA-9 in a subset of schizophrenia patient-derived
neural progenitor cells, Cell Rep., 15, 1024-1036.
50.Murai, K., Sun, G., Ye, P., Tian, E., Yang, S.,
Cui, Q., Sun, G., Trinh, D., Sun, O., Hong, T., Wen, Z., Kalkum, M.,
Riggs, A. D., Song, H., Ming, G. L., and Shi, Y. (2016) The TLX-miR-219
cascade regulates neural stem cell proliferation in neurodevelopment
and schizophrenia iPSC model, Nat. Commun., 7, 10965.
51.Dugas, J. C., Cuellar, T. L., Scholze, A., Ason,
B., Ibrahim, A., Emery, B., Zamanian, J. L., Foo, L. C., McManus, M.
T., and Barres, B. A. (2010) Dicer1 and miR-219 are required for normal
oligodendrocyte differentiation and myelination, Neuron,
65, 597-611.
52.Lerner, R. A., and Tramontano, A. (1987)
Antibodies as enzymes, Trends Bioch. Sci., 12,
427-438.
53.Schultz, P. G., and Lerner, R. A. (1995) From
molecular diversity to catalysis: lessons from the immune system,
Science, 269, 1835-1842.
54.Keinan, E. (ed.) (2005) Catalytic
Antibodies, Wiley-VCH Verlag GmbH and Co. KgaA, Weinheim,
Germany.
55.Nevinsky, G. A., and Buneva, V. N. (2005) Natural
catalytic antibodies – abzymes, in Catalytic Antibodies
(Keinan, E., ed.), VCH-Wiley Press, Weinheim, Germany, pp. 505-569.
56.Nevinsky, G. A. (2010) Natural catalytic
antibodies in norm and in autoimmune diseases, in Autoimmune
Diseases: Symptoms, Diagnosis and Treatment (Brenner, K. J.,
ed.), Nova Science Publishers, Inc., New York, USA.
57.Nevinsky, G. A. (2011) Natural catalytic
antibodies in norm and in HIV-infected patients, in Understanding
HIV/AIDS Management and Care – Pandemic Approaches in the 21st
Century (Kasenga, F. H., ed.), InTech, Rijeka, Croatia, pp.
151-192.
58.Nevinsky, G. A. (2016) Autoimmune processes in
multiple sclerosis: production of harmful catalytic antibodies
associated with significant changes in the hematopoietic stem cell
differentiation and proliferation, in Multiple Sclerosis
(Conzalez-Quevedo, A., ed.), InTech, Rijeka, Croatia, pp. 100-147.
59.Andryushkova, A. A., Kuznetsova, I. A., Buneva,
V. N., Toporkova, L. B., Sakhno, L. V., Tikhonova, M. A., Chernykh, E.
R., Orlovskaya, I. A., and Nevinsky, G. A. (2007) Formation of
different abzymes in autoimmune‐prone MRL‐lpr/lpr mice is
associated with changes in colony formation of hematopoietic
progenitors, J. Cell. Mol. Med., 11, 531-551.
60.Buneva, V. N., Krasnorutskiy, M. A., and
Nevinskiy, G. A. (2013) Natural antibodies against nucleic acids,
Biochemistry (Moscow), 78, 127-143.
61.Ermakov, E. A., Smirnova, L. P., Parkhomenko, T.
A., Dmitrenok, P. S., Krotenko, N. M., Fattakhov, N. S., Bokhan, N. A.,
Semke, A. V., Ivanova, S. A., Buneva, V. N., and Nevinsky, G. A. (2015)
DNA-hydrolysing activity of IgG antibodies from the sera of patients
with schizophrenia, Open Biol., 5, 150064.
62.Parshukova, D. A., Smirnova, L. P., Buneva, V.
N., Semke, A. V., and Ivanova, S. A. (2015) Proteolytic hydrolysis of
myelin basic protein by IgGs during long-term treatment of
schizophrenia, Eur. Neuropsychopharmacol., 25
S272-S273.
63.Baranovskiy, A. G., Kanyshkova, T. G.,
Mogil’nitskiy, A. S., Naumov, V. A., Buneva, V. N., Boyko, A. N.,
Favorova, O. O., and Nevinskiy, G. A. (1998) Polyclonal serum
antibodies in patients with multiple sclerosis efficiently hydrolyzed
RNA and DNA, Biochemistry (Moscow), 63,
1239-1248.
64.Vlasov, A. V., Baranovskiy, A. G., Kanyshkova, T.
G., Prints, A. V., Zabara, V. G., Naumov, V. A., Breusov, A. A., Giege,
R., Buneva, V. N., and Nevinskiy, G. A. (1998) Substrate specificity of
DNA- and RNA-hydrolyzing serum antibodies from patients with
polyarthritis and autoimmune thyroiditis, Mol. Biol. (Moscow),
32, 559-569.
65.Vlassov, A., Florentz, C., Helm, M., Naumov, V.,
Buneva, V., Nevinsky, G., and Giege, R. (1998) Characterization and
selectivity of catalytic antibodies from human serum with RNase
activity, Nucleic Acids Res., 26, 5243-5250.
66.Nevinsky, G. A., and Buneva, V. N. (2002) Human
catalytic RNA- and DNA-hydrolyzing antibodies, J. Immunol.
Methods, 269, 235-249.
67.Kay, S. R., Abraham, F., and Lewis, A. O. (1987)
The positive and negative syndrome scale (PANSS) for schizophrenia,
Schizophrenia Bull., 13, 261.
68.Kneisl, C., and Trigoboff, E. (2009)
Contemporary Psychiatric-Mental Health Nursing, 2nd Edn.,
Pearson Prentice Ltd., London.
69.American Psychiatric Association. Task Force on
DSM-IV (2000) Diagnostic and Statistical Manual of Mental Disorders
(DSM), 4th Edn., American Psychiatric Association, Washington,
DC.
70.Carson, V. B. (1995) Mental Health Nursing:
The Nurse–Patient Journey, 2nd Edn., W. B. Saunders,
Philadelphia.
71.Velligan, D. I., and Alphs, L. D. (2008) Negative
symptoms in schizophrenia: the importance of identification and
treatment, Psychiat. Times, 25, 12.
72.Polosukhina, D. I., Kanyshkova, T. G., Doronin,
B. M., Tyshkevich, O. B., Buneva, V. N., Boiko, A. N., Gusev, E. I.,
Favorova, O. O., and Nevinsky, G. A. (2004) Hydrolysis of myelin basic
protein by polyclonal catalytic IgGs from the sera of patients with
multiple sclerosis, J. Cell. Mol. Med., 8, 359-368.
73.Polosukhina, D. I., Buneva, V. N., Doronin, B.
M., Tyshkevich, O. B., Boiko, A. N., Gusev, E. I., Favorova, O. O., and
Nevinsky, G. A. (2006) Metal-dependent hydrolysis of myelin basic
protein by IgGs from the sera of patients with multiple sclerosis,
Immunol. Lett., 103, 75-81.
74.Bezuglova, A. M., Konenkova, L. P., Doronin, B.
M., Buneva, V. N., and Nevinsky, G. A. (2011) Affinity and catalytic
heterogeneity and metal‐dependence of polyclonal myelin basic
protein‐hydrolyzing IgGs from sera of patients with systemic
lupus erythematosus, J. Mol. Recognit., 24, 960-974.
75.Krasnorutskii, M. A., Buneva, V. N., and
Nevinsky, G. A. (2008) Antibodies against pancreatic ribonuclease A
hydrolyze RNA and DNA, Int. Immunol., 20, 1031-1040.
76.Andrievskaya, O. A., Buneva, V. N., Baranovskii,
A. G., Gal’vita, A. V., Benzo, E. S., Naumov, V. A., and
Nevinsky, G. A. (2002) Catalytic diversity of polyclonal
RNA-hydrolyzing IgG antibodies from the sera of patients with systemic
lupus erythematosus, Immunol. Lett., 81, 191-198.
77.Fisher, B. M., Juneko, E. G., and Ronald, T. R.
(1998) A new remote subsite in ribonuclease A, J. Biol. Chem.,
273, 34134-34138.
78.Irie, M., Mikami, F., Monma, K., Ohgi, K.,
Watanabe, H., Yamaguchi, R., and Nagase, H. (1984) Kinetic studies on
the cleavage of oligouridylic acids and poly U by bovine pancreatic
ribonuclease A, J. Biochem., 96, 89-96.
79.Crook, E. M., Mathias, A. P., and Rabin, B. R.
(1960) Spectrophotometric assay of bovine pancreatic ribonuclease by
the use of cytidine 2′:3′-phosphate, Biochem. J.,
74, 234-238.
80.Fersht, A. (1985) Enzyme Structure and
Mechanism, 2nd Edn., W. H. Freeman, Co., New York.
81.Akagi, K., Murai. K., Hirao, N., and Yamanaka, M.
(1976) Purification and properties of alkaline ribonucleases from human
serum, Biochim. Biophys. Acta, 442, 368-378.
82.Tolmacheva, A. S., Blinova, E. A., Ermakov, E.
A., Buneva, V. N., Vasilenko, N. L., and Nevinsky, G. A. (2015) IgG
abzymes with peroxidase and oxidoreductase activities from the sera of
healthy humans, J. Mol. Recognit., 28, 565-580.
83.Andreev, S. L., Buneva, V. N., and Nevinsky, G.
A. (2016) How human IgGs against DNA recognize oligonucleotides and
DNA, J. Mol. Recognit., 29, 596-610.
84.Andrievskaya, O. A., Buneva, V. N., Naumov, V.
A., and Nevinsky, G. A. (2000) Catalytic heterogeneity of polyclonal
RNA-hydrolyzing IgM from sera of patients with lupus erythematosus,
Med. Sci. Monit., 6, 460-470.
85.Pollak, T. A., Beck, K., Irani, S. R., Howes, O.
D., David, A. S., and McGuire, P. K. (2016) Autoantibodies to central
nervous system neuronal surface antigens: psychiatric symptoms and
psychopharmacological implications, Psychopharmacology (Berl.),
233, 1605-1621.
86.Andryushkova, A. S., Kuznetsova, I. A.,
Orlovskaya, I. A., Buneva, V. N., and Nevinsky, G. A. (2006) Antibodies
with amylase activity from the sera of autoimmune-prone MRL/MpJ-lpr
mice, FEBS Lett., 580, 5089-5095.
87.Andryushkova, A. A., Kuznetsova, I. A.,
Orlovskaya, I. A., Buneva, V. N., and Nevinsky, G. A. (2009)
Nucleotide-hydrolyzing antibodies from the sera of autoimmune-prone
MRL-lpr/lpr mice, Int. Immunol., 21, 935-945.
88.Doronin, V. B., Parkhomenko, T. A., Korablev, A.,
Toporkova, L. B., Lopatnikova, J. A., Alshevskaja, A. A., Sennikov, S.
V., Buneva, V. N., Budde, T., Meuth, S. G., Orlovskaya, I. A., Popova,
N. A., and Nevinsky, G. A. (2016) Changes in different parameters,
lymphocyte proliferation and hematopoietic progenitor colony formation
in EAE mice treated with myelin oligodendrocyte glycoprotein, J.
Cell. Mol. Med., 20, 81-94.
89.Aulova, K. S., Toporkova, L. B., Lopatnikova, J.
A., Alshevskaya, A. A., Sennikov, S. V., Buneva, V. N., Budde, T.,
Meuth, S. G., Popova, N. A., Orlovskaya, I. A., and Nevinsky, G. A.
(2017) Changes in hematopoietic progenitor colony differentiation and
proliferation and the production of different abzymes in EAE mice
treated with DNA, J. Cell. Mol. Med., 21, 3795-3809.
90.Parkhomenko, T. A., Doronin, V. B., Castellazzi,
M., Padroni, M., Pastore, M., Buneva, V. N., Granieri, E., and
Nevinsky, G. A. (2014) Comparison of DNA-hydrolyzing antibodies from
the cerebrospinal fluid and serum of patients with multiple sclerosis,
PLoS One, 9, e93001.
91.Doronin, V. B., Parkhomenko, T. A., Castellazzi,
M., Padroni, M., Pastore, M., Buneva, V. N., Granieri, E., and
Nevinsky, G. A. (2014) Comparison of antibodies hydrolyzing myelin
basic protein from the cerebrospinal fluid and serum of patients with
multiple sclerosis, PLoS One, 9, e107807.
92.Doronin, V. B., Parkhomenko, T. A., Castellazzi,
M., Cesnik, E., Buneva, V. N., Granieri, E., and Nevinsky, G. A. (2016)
Comparison of antibodies with amylase activity from cerebrospinal fluid
and serum of patients with multiple sclerosis, PLoS One,
11, e0154688.