Implication of Integrin α2β1 in Proliferation and Invasion of Human Breast Carcinoma and Melanoma Cells: Noncanonical Function of Akt Protein Kinase
N. I. Kozlova1, G. E. Morozevich1, N. A. Ushakova1, and A. E. Berman1,a*
1Orekhovich Institute of Biomedical Chemistry, Russian Academy of Sciences, 119121 Moscow, Russia* To whom correspondence should be addressed.
Received January 10, 2018; Revision received February 26, 2018
Blocking the expression of integrin α2β1, which was accomplished by transduction of α2-specific shRNA, resulted in significant inhibition of proliferation and clonal activity in human MCF-7 breast carcinoma and SK-Mel-147 melanoma cells. Along with these changes, deprivation of α2β1 caused a sharp decrease in melanoma cell invasion in vitro. Analysis of integrin-mediating signal pathways that control cell behavior revealed a significant increase in activity of Akt protein kinase in response to depletion of α2β1. The increase in Akt activity that accompanies a suppressive effect on cell invasion contradicts well-known Akt function aimed at stimulation of tumor progression. This contradiction could be explained by the “reversed” (noncanonical) role played by Akt in some cells that consists in suppression rather than promotion of invasive phenotype. To test this suggestion, the effects of Akt inhibitors on invasive activity of SK-Mel-147 cells were investigated. If the above suggestion is true, then inhibition of Akt in cells depleted of α2β1 should result in the restoration of their invasive activity. It appeared that treatment with LY294002, which inhibits all Akt isoforms (Akt1, Akt2, Akt3), not only failed to restore the invasive phenotype of melanoma cells but further attenuated their invasive activity. However, treatment of the cells with an Akt1-specific inhibitor significantly increased their invasion. Thus, the stimulating effect of α2β1 integrin on invasion of melanoma cells is realized through a mechanism based on inhibition of one of the Akt isoforms, which in these cells exhibits a noncanonical function consisting in suppression of invasion.
KEY WORDS: integrins, tumor growth, proliferation, invasion, Akt protein kinase, signalingDOI: 10.1134/S0006297918060111
Abbreviations: ECM, extracellular matrix; EdU, 5-ethynyl-2′-deoxyuridine.
Malignant transformation of cells and tumor progression is mainly
controlled by the extracellular matrix (ECM). This control occurs via
signal transduction (signaling), a successive trafficking of the matrix
generated signals delivered to nuclei through a chain of intracellular
molecules with subsequent modification of gene activity. Key mediators
in signal transduction are the integrins, cell surface receptors that
directly associate with the matrix proteins and initiate signaling.
Numerous experimental data indicate that integrins are involved in the
basic behavioral responses of cells (proliferation, differentiation,
mobility, apoptosis) whose modifications are the basis of tumor
progression [1-4].
Integrins comprise a large family of about 20 members, each being a heterodimer consisting of α- and β-chains connected by noncovalent bonds. Integrins markedly differ from each other in ligand specificity and expression levels in mammalian tissues. The most common and vital for mammalian cells are fibronectin-binding integrin α5β1, the collagen-binding receptor α2β1, and integrin αvβ3 having a diverse ligand specificity. These receptors have been the subject of the majority of studies aimed at clarifying the role of integrin-mediated signaling in growth and progression of tumors [5-7]. Data on the functions of individual integrins are controversial. This may result because of the diversity of the integrin family and the variety of signal pathways that a particular receptor can induce in different cells. In mice transgenic for the human papilloma virus (HPV), deletion of the α2 integrin gene did not affect the growth of HPV-induced squamous carcinoma, while it significantly increased metastasis in regional lymph nodes [8]. These results are in line with the observation of a significant contribution of α2β1 to progression of melanoma, hepatoma, and lung carcinoma cells [9-11]. However, in a mouse model of spontaneous breast cancer, α2β1 suppressed metastatic dissemination [12]. As mentioned above, these contradictions may result from differences between distinct tumor histotypes in signaling pathways initiated by integrin α2β1 [13-16].
In this study, it has been shown that downregulation of α2β1 in human breast carcinoma and melanoma cells strongly suppresses their malignant phenotype, in particular, the in vitro invasion activity of melanoma cells. We demonstrated for the first time that a stimulating effect of α2β1 integrin on melanoma cell invasion is realized through a noncanonical mechanism based on inhibition, rather than stimulation, of the Akt1 isoform of Akt protein kinase.
MATERIALS AND METHODS
Cells and reagents. MCF-7 human breast cancer cell line was obtained from the ATCC (American Type Culture Collection, USA). SK-Mel-147 human melanoma line, previously shown to be highly metastatic [17], was obtained from the Memorial Sloan Kettering Cancer Center, USA. Cells were cultured in DMEM medium containing 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated at 37°C in an atmosphere with 5% CO2.
Polyclonal antibodies against α2-integrin subunit and monoclonal antibodies against α2β1 integrin were obtained from Chemicon and BD Pharmingen (USA), respectively. Polyclonal antibodies against protein kinases Akt and Erk and their phosphorylated forms (pAkt Ser473 and pErk Thr202/Tyr204) were from Cell Signaling Tech (USA). Erk inhibitor (PD98059), PI3K/Akt inhibitor (LY294002), Akt1- and Akt2-specific inhibitors (XXIII and XII, respectively) were purchased from Calbiochem (USA), and other chemicals were from Sigma (USA) unless otherwise stated.
Cell transduction with shRNA. Bacterial glycerol clones NM_002203.2-1427s1c1 (#D3) and NM_002203.3-1561s21c1 (#D7) containing lentiviral plasmid vector pLKO.1-puro with shRNA specific for the α2-integrin subunit and pLKO.1-puro lentiviral vector without shRNA (“empty” vector, control) were purchased from Sigma. The preparation of lentiviral particles and infection of the cells was performed as described previously [18].
Proliferation and DNA synthesis. Cells (2·104) were seeded in DMEM supplemented with 10% fetal serum in 96-well culture plates. After distinct time intervals, the cells were stained with Crystal Violet and dissolved in 1% SDS, and then optical density was determined at 570 nm. DNA synthesis was assayed by the incorporation of EdU (5-ethynyl-2′-deoxyuridine) followed by staining of replicating and total DNA, as described in the manufacturer’s protocol (Invitrogen, USA). Briefly, the cells in the logarithmic growth phase were seeded on coverslips, incubated at 37°C for 1 h with 10 μM EdU, fixed with 3.7% formaldehyde, permeabilized by Triton X-100 (0.5%) treatment, stained with Alexa Fluor 488 and Hoechst 33342, and viewed in a luminescence microscope at wavelengths (excitation/emission, nm) of 495/519 for Alexa Fluor 488 and 350/461 for Hoechst 33342.
Invasion in vitro. The assays were performed as previously described [19] with minor modifications. Cells ((5-7)·104) in 150 μl DMEM containing 0.5% fetal serum were applied on Matrigel (BD Pharmingen, USA) immobilized on 8 μm-pore membranes of the upper chamber of Transwell clusters (Corning, USA) with the bottom chambers containing 1 ml of the same medium. After 48-72 h at 37°C in an atmosphere of 5% CO2, the number of cells that migrated from the upper to the bottom chamber was counted.
Clonal activity. About 2000 cells were plated on a 1% methylcellulose gel in complete medium in Petri dishes for 14 days. The colonies were stained with Crystal Violet, visualized in an optical microscope, and scanned.
PAGE and immunoblotting. Cells were extracted with buffer (50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitor cocktails (Santa Cruz Biotech, USA), each 1 μl/106 cells, and centrifuged at 13,000g for 10 min. Further treatments were performed as described [20].
FACScan analysis was performed as previously described [20].
Statistical analysis. Differences between groups were assessed using Student’s t-test. Significance level was set at p < 0.05.
RESULTS
Downregulation of integrin α2β1 signaling inhibits proliferation and clonal activity of tumor cells. Signal activity of integrin α2β1 was blocked by suppressing its expression with the α2-specific shRNA. In this study, we explored two plasmid clones containing α2-specific shRNA. In our previous investigation, both clones were shown to be highly efficient in downregulation of α2β1 expression in MCF-7 breast carcinoma cells [20]. Analysis of its expression in SK-Mel-147 melanoma cells transduced with these clones also demonstrated their high efficacy, as indicated by the fact that the expression of α2β1 in cells transduced with any of them was 2-2.5 times lower than in cells transduced with an empty plasmid (FACScan data, not shown). Subsequent studies were performed with the D3 clone, which, like in the MCF-7 cells, was more effective.
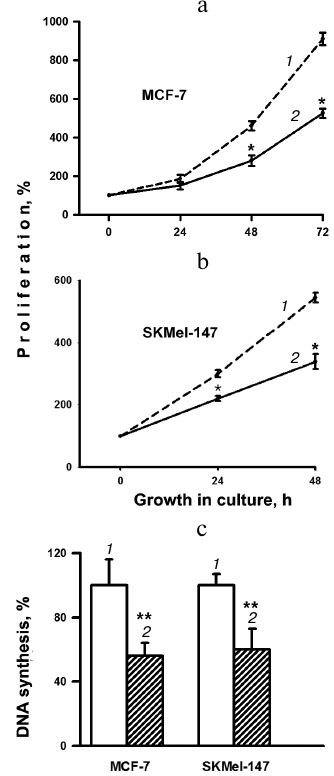
Proliferative activity was assessed by the change in cell growth in culture for 0-72 h (Fig. 1, a and b). In the MCF-7 line, after 24 h of growth, there was a marked decrease in the number of cells transduced with α2 shRNA compared to cells transduced with the control vector. After 48 h of growth, the number of cells in the experimental samples of the MCF-7 line decreased by ~40% compared to the control. Almost the same decrease in growth was detected in SK-Mel-147 experimental samples compared to the controls. These results are consistent with data on the change in DNA synthesis in response to blocking the expression of integrin α2β1 (Fig. 1c). During 1-h incubation with EdU, both lines showed almost the same decrease (46 and 40%) in the amount of EdU incorporation in cells depleted of α2β1 compared to the control cells.
Fig. 1. Effect of downregulation of integrin α2β1 expression on proliferation (a, b) and DNA synthesis (c) in human MCF-7 breast carcinoma and SK-Mel-147 melanoma cells. a, b) The cells were transduced with PLKO.1 plasmid vector containing α2-specific shRNA or with the empty vector (control) and treated as described in “Materials and Methods”. c) Cells in logarithmic growth phase were incubated with EdU and stained as described in “Materials and Methods”, and then the ratio of the number of Alexa Fluor 488-stained cells to that of cells stained with Hoechst 33342 was determined. The ratio determined for control cells was taken as 100%; 1) cells transduced with the empty vector; 2) cells transduced with α2 shRNA-containing vector. The results of three independent experiments are shown (M ± SEM); * p < 0.05 relative to (1); ** p < 0.02 relative to (1).
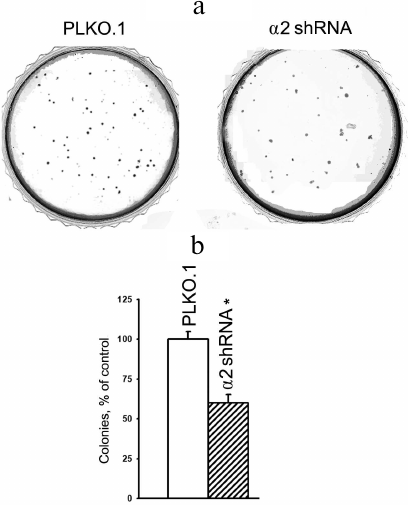
An intrinsic feature of malignant transformation is capacity of tumor cells to grow in the absence of ECM contacts and to form colonies in semi-liquid media (agarose, methylcellulose). Normal cells detached from the ECM, undergo a kind of apoptosis termed anoikis. The acquisition by tumor cells of ability to overcome anoikis is largely determined by cell surface molecules, including integrins. We have shown previously that MCF-7 cells reduced by ~3-fold their ability to form colonies in a methylcellulose gel in response to α2β1 knock-down [20]. In SK-Mel-147 cells, clonal activity also diminished in response to α2β1 suppression, although to a less degree than in MCF-7 cells (Fig. 2).
Fig. 2. Effect of α2β1 downregulation on clonal activity of SK-Mel-147 cells. Cells transduced with the empty (PLKO.1) or α2 shRNA-containing vector were grown in semiliquid methylcellulose for 14 days as described in “Materials and Methods”. Colonies on Petri dishes were stained with Crystal Violet and scanned, and the number of colonies was counted. The number of colonies formed by control cells was taken as 100%. a) The result of a typical experiment is shown; b) results of three independent experiments are shown (M ± SEM); * p < 0.02, relative to PLKO.1.
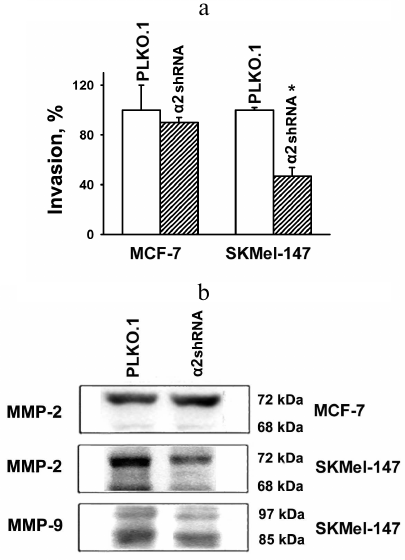
Blockade of α2β1 signaling inhibits invasive activity of SK-Mel-147 cells. As mentioned, numerous data indicate that the modes and ways of α2β1 involvement in mechanisms of tumor progression, especially in degradation and invasion of the ECM, are contradictory and depend on cell histotypes and features of cell lines. In the present study, we analyzed an invasive activity of two quite different tumor lines using an in vitro invasion model: the cells were assessed for their ability to induce destruction and invasion of Matrigel, a gel reconstructed from basement membrane proteins. In addition, the expression of matrix-specific proteases (MMP-2 and MMP-9) was determined, since increase in their activity is one of the characteristic feature of invasive phenotype. It appeared that MCF-7 cells are virtually inactive in in vitro invasion, and transduction of α2-specific shRNA does not affect their invasive activity (Fig. 3a). This result corroborates low metastatic activity of these cells [21]. On the other hand, MCF-7 cells were found to be active in synthesis of MMP-2 collagenase, which is mainly represented by an inactive form (with molecular weight of 72 kDa), while the active form (with molecular weight 68 kDa) was not detected. Unlike the MCF-7 line, highly metastatic SK-Mel-147 cells demonstrated not only enhanced MMP-2 expression but also a visible increase in the level of active MMP-2 form (Fig. 3b). Besides, in contrast to MCF-7, melanoma cells were highly active in expression of active (85 kDa) MMP-9 (Fig. 3b). One can see that downregulation of α2β1 led to a significant decrease in active and inactive MMP-9 forms in melanoma cells, a finding that is in line with the fact that the depletion of these cells of α2β1 resulted in about 2-fold decrease in their invasive activity (Fig. 3a).
Fig. 3. Effect of α2β1 downregulation on in vitro invasion (a) and activity of matrix metalloproteinases (b) in MCF-7 and SK-Mel-147 cells. a) Cells transduced with the control (PLKO.1) or α2 shRNA-containing vectors were applied to Matrigel, and the number of cells migrated into the bottom chamber of transwell clusters was counted (see “Materials and Methods”). Invasion was calculated as ratio (%) of the number of migrated α2 shRNA-transduced cells to that of empty vector-transduced cells. Results of three independent experiments are shown (M ± SEM); * p < 0.02, relative to PLKO.1. b) Immunoblotting of cell lysate proteins. Cell lysate proteins (30 µg) were run on 7.5% SDS-PAGE, electroblotted onto a PVDF membrane, probed with primary and secondary antibodies, and analyzed in a gel- and chemi-documentation system.
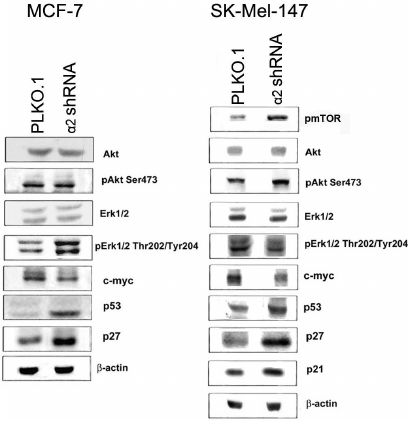
Signal pathways mediating the effects of α2β1 downregulation. To clarify the mechanisms mediating the effect of α2β1 integrin on invasion, we assessed the expression of proteins that participate in signal pathways and control the physiological functions of cells. Immunoblot analysis of cell lysate proteins (obtained from control and α2 shRNA-transduced cells) showed that downregulation of α2β1 resulted in a dramatic increase in expression of apoptogenic p53 protein in SK-Mel-147 cells (Fig. 4). In addition, the expression of cell cycle inhibitors, proteins p21 and p27, was significantly increased, while c-myc protein expression was reduced. These proteins perform important functions in the mechanisms of cell proliferation and survival [22, 23]. Similar changes in these proteins were detected earlier in the MCF-7 line [20]. Along with these characteristics, we assessed the impact of α2β1 downregulation on expression and activity of Erk1/2 protein kinase (Erk isomers with molecular weights of 42 and 44 kDa) and Akt kinase, both of which play a key role in transmitting integrin-induced signals. Expression and activity of these enzymes were determined by immunoblot analysis of cell lysate proteins using antibodies to kinase total protein and to its active (phosphorylated) forms. As shown in Fig. 4, decrease in α2β1 did not affect the content of both kinases nor the activity of Erk1/2 kinase, but was accompanied by a significant increase in activity of Akt kinase in SK-Mel-147 cells. In contrast, in MCF-7 cells, downregulation of α2β1 had no effect on Akt activity while it enhanced the activity of Erk1/2 [20].
Fig. 4. Effect of α2β1 integrin knockdown on expression of signaling proteins in MCF-7 and SK-Mel-147 cells. Cell lysate proteins (30 µg) from cells transduced with empty vector or α2 shRNA-containing vector were run on 7.5% SDS-PAGE, followed by immunoblotting as specified in “Materials and Methods”. The membrane was probed with antibodies to the indicated proteins at a 1 : 1000 dilution (see legend to Fig. 3).
Noncanonical function of Akt1 in invasive phenotype of SK-Mel-147 cells. Enhanced Akt activity found in SK-Mel-147 cells with decreased invasive potential might result from noncanonical Akt functions in these cells, consisting in suppressing cell growth and progression, in contrast to well-known Akt function of promoting tumor progression documented in various cell types [24, 25]. But it could not be excluded that the observed increase in Akt activity is a sign accompanying downregulation of α2β1 in SK-Mel-147 cells and is not related to mechanisms controlling tumor progression. To address this question, the effect of Akt inhibition on proliferation and invasive activity of SK-Mel-147 cells was assessed. We hypothesized that if inhibition of melanoma cell proliferation induced by α2β1 knockdown occurs via activation of the PI3K/Akt signal pathway, inhibition of this kinase should neutralize the inhibitory effect or even enhance proliferation. It was found, however, that treating α2 shRNA-transduced cells with the specific PI3K/Akt inhibitor LY294002 leads to further inhibition of cell proliferation (data not shown).
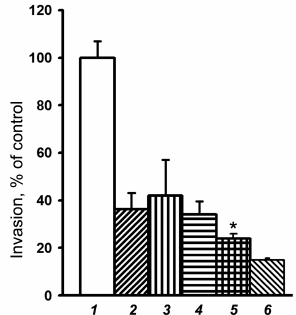
Similar results were obtained when investigating the effect of LY294002 on invasion of melanoma cells. As shown in Fig. 5, empty vector-transduced melanoma cells, upon the treatment with LY294002, reduced their invasion activity by 2.5 times, i.e. to the level of the α2 shRNA-transduced cells, not treated with this inhibitor. Thus, these data are not in line with the suggestion that Akt can suppress invasive activity in the studied melanoma cells.
Fig. 5. Effect of Akt and Erk protein kinase inhibitors on in vitro invasion of SK-Mel-147 cells. Cells transduced with control (1-3) or α2 shRNA-containing (4-6) vectors were treated for 24 h with 25 µM Akt inhibitor, LY294002 (2, 5), or 25 µM Erk inhibitor, PD98059 (3, 6), followed by assaying in vitro invasion (see “Materials and Methods”). Invasion of control vector-transduced cells not treated with the inhibitors (column 1) was taken as 100%. The results of three independent experiments are shown (M ± SEM); * p < 0.05 relative to (4).
It is known that Akt kinase is represented by three isoforms (Akt1, Akt2, Akt3) that differ in expression level in distinct cell types of different origin [26]. Several studies have also demonstrated significant differences between individual isoforms in their effect on behavioral cellular responses, including metastatic and invasive activity of tumor cells. For example, suppression of Akt1 stimulated proliferation and epithelial–mesenchymal transition (EMT) of mammary epithelial cells, while downregulation of Akt2 eliminated this effect [27]. Notably, LY294002 does not exhibit isozyme-specific effects and suppresses the activity of all isoforms. One could speculate that increased level of phosphorylated Akt found in α2β1-depleted melanoma cells is the result of activation of one of the Akt isoforms, which unlike the other two inhibits invasion, i.e. manifests a noncanonical function. In our previous investigation we have shown that blockade of Akt1 significantly attenuated anchorage-dependent apoptosis (anoikis) in SK-Mel-147 cells, while inhibition of two other isoforms stimulated anoikis (in press).
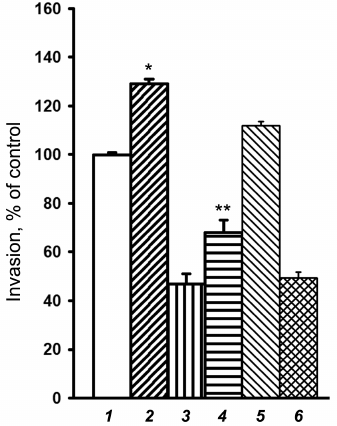
In view of these results, we analyzed the effect of Akt1 inhibition on in vitro invasion of melanoma cells transduced with α2-specific shRNA. As shown in Fig. 6, the Akt1-specific inhibitor XXIII exerted a pronounced stimulating effect on invasion of the studied cells, independent of their α2β1 expression. This indicates that the Akt1 isoform can reduce invasive potential of SK-Mel-147 melanoma cells. It is also seen that inhibition of Akt2 by the specific inhibitor XII did not have a noticeable effect on the invasion of control and α2 shRNA-transduced cells. The effect of Akt3 on invasion of SK-Mel-147 cells is now being analyzed.
Fig. 6. Effect of inhibitors of Akt isoforms on in vitro invasion of SK-Mel-147 cells. Cells transduced with control (1, 2, 5) or α2 shRNA-containing vector (3, 4, 6) were treated for 24 h with 3 µM Akt1-specific inhibitor XXIII (2, 4) or 5 µM Akt2-specific inhibitor XII (5, 6) followed by invasion assaying (see “Materials and Methods”). Invasion of control vector-transduced cells not treated with the inhibitors (column 1) was taken as 100%. The results of three independent experiments are shown (M ± SEM); * p < 0.02 relative to (1); ** p < 0.05 relative to (3).
In conclusion, we have provided direct evidence that in regulating tumor progression at least one of the Akt isoforms can perform functions that are not typical to this key signal molecule. Another conclusion is that impact of α2β1 integrin on tumor progression can be mediated through a noncanonical activity of Akt.
DISCUSSION
As noted, data on the role of the α2β1 receptor in tumor progression (invasion, metastasis) are contradictory. In addition to the cited data reported by Tran et al. [8], it should be mentioned the work by Dyce et al. [28] demonstrating that mouse xenografts of a human squamous cell carcinoma line with high α2β1 expression exhibited more pronounced invasion into surrounding tissues compared to the lines with low α2β1 expression. A similar tendency was observed by Sottnik et al. [29], who showed that overexpression of integrin α2β1 stimulates, while its downregulation inhibits, adhesion and migration of prostate cancer cells on collagen. In addition, deficiency of α2β1 reduced bone metastasis of prostate cancer cells inoculated into animals. A key role of α2β1 integrin in metastasizing was demonstrated in studies on bone metastasis developed in mice grafted with B16 mouse melanoma cells [30].
However, as reported by Ramirez et al., deletion of the α2 integrin in MMTV-Neu (mouse mammary tumor virus) infected mice, characterized by a high breast tumor incidence, led to a drastic increase in spontaneous lung metastasis estimated by the number and size of metastases [12]. On the other hand, they demonstrated that cell lines derived from primary breast tumors with high α2 gene expression did not differ in proliferative activity from the lines derived from tumors devoid of α2 gene expression.
Our results demonstrating that loss of α2β1 expression led to a decrease in active MMP-2 corroborate the cited investigation. However, we have shown that α2β1 downregulation inhibited proliferation of the cells with low metastatic potential while it reduced both proliferation and invasion of cells with high metastatic activity. Further research is needed to clarify these contradictions. Of a principal interest is our data indicating the ability of one Akt isoform to induce signals aimed at attenuating rather than promoting invasive activity of tumor cells. Importantly, this noncanonical function of one of the key signal molecules was shown to be implicated in a mechanism by which integrins can control the invasive activity of tumor cells – the most important property of a malignant phenotype. This observation made on a melanoma line is consistent with studies on the other tumor histotypes. For example, in a breast adenocarcinoma line with high metastatic potential, the effect of TIS21 tumor suppressor, which consists in inhibiting the formation of invadopodia and, therefore, blocking the invasion, is realized through a mechanism mainly based on activation of Akt1, while changes in Akt2 expression had no impact on invadopodia formation and invasive activity [31]. The ambivalent properties of Akt isoforms have been documented in several investigations. Akt1 was shown to stimulate the growth of breast carcinoma but inhibit its metastasis [32]. In contrast, Akt2 enhanced invasion and metastasis of breast and ovarian carcinoma cells [33]. This finding has recently been confirmed on cells of the same origin [34]. It can be assumed that distinct cellular types differ in the expression of individual Akt isoforms, and therefore their responses to Akt-mediated signaling may be different.
Signaling pathways mediating inhibitory effect of Akt1 on tumor progression are diverse, and, according to published information, depend on tumor origin. The diversity is typical for the upstream signals activating Akt1, as well as for downstream Akt1-induced effectors that modify cell behavior. In the cited investigation [31] on a highly invasive breast carcinoma line, tumor suppressor TIS21 by activating Akt1 triggers a signal chain that is completed by depolymerization of F-actin filaments, regression of lamello- and invadopodia, and suppression of invasion. In more detail, activation of Akt1 results in its translocation to nucleus with subsequent binding and inactivation of SP1 (Nox4-specific transcription factor) causing inhibition of Nox4 (NADPH-oxidase) transcription. Reduced Nox4 level leads to a decrease in reactive oxygen species (ROS) production, and the resulting ROS deficiency downregulates the expression of diaphanous-related (DR)-formin responsible for F-actin polymerization.
An alternative signal pathway was identified in a non-small cell lung cancer line in which antimetastatic effect of Akt1 is accomplished via inactivation of MARCKS, a protein kinase C substrate that binds actin and regulates its polymerization [35]. But the mechanisms underlying the effect of Act1 on MARCKS remains unclear. Intriguing in this investigation is a feedback found between LAMC2, a protein known to stimulate motility and invasion of tumor cells, and Akt1. The authors showed that downregulation of Akt1 increases the expression of LAMC2, while enhanced level of this protein amplifies the inhibitory effect on Akt1 and reinforces cell invasion.
From the point of the role of integrin–Akt interactions in tumor progression, of considerable interest are the results demonstrating that the anti-invasive activity of Akt1 in a breast carcinoma line is accounted for by the inhibitory effect on expression of β1-family integrins and on their downstream effector, protein kinase FAK [34]. However, in these cells, unlike their above-mentioned close relatives [31], anti-invasive effect of Akt1 was not accompanied by remodeling of actin filaments. In this regard, it remains unclear what morphological changes in the cell surface ensures the acquisition of an invasive phenotype by the cells.
Acknowledgments
This study was performed in the framework of the Program for Basic Research of State Academies of Sciences for 2013-2020 and supported by the Russian Foundation for Basic Research (grant No. 17-04-00716).
REFERENCES
1.Guo, W., and Giancotti, F. G. (2004) Integrin
signalling during tumour progression, Nat. Rev. Mol. Cell.
Biol., 10, 816-826.
2.Hehlgans, S., Haase, M., and Cordes, N. (2007)
Signalling via integrins: implications for cell survival and anticancer
strategies, Biochim. Biophys. Acta, 1775, 163-180.
3.Desgrosellier, J. S., and Cheresh, D. A. (2010)
Integrins in cancer: biological implications and therapeutic
opportunities, Nat. Rev. Cancer, 10, 9-22.
4.Kuphal, S., Bauer, R., and Bosserhoff, A. K. (2005)
Integrin signaling in malignant melanoma, Cancer Metastasis
Rev., 24, 195-222.
5.Haenssen, K. K., Caldwell, S. A., Shahriari, K. S.,
Jackson, S. R., Whelan, K. A., Klein-Szanto, A. J., and Reginato, M. J.
(2010) ErbB2 requires integrin alpha 5 for anoikis resistance via Src
regulation of receptor activity in human mammary epithelial cells,
J. Cell Sci., 123, 1373-1382.
6.Bai, J., Zhang, J., Wu, J., Shen, L., Zeng, J.,
Ding, J., Wu, Y., Gong, Z., Li, A., Xu, S., Zhou, J., and Li, G. (2010)
JWA regulates melanoma metastasis by integrin alphaVbeta 3 signaling,
Oncogene, 29, 1227-1237.
7.Nam, E. H., Lee, Y., Moon, B., Lee, J. W., and Kim,
S. (2015) Twist1 and AP-1 cooperatively upregulate integrin α5
expression to induce invasion and the epithelial–mesenchymal
transition, Carcinogenesis, 36, 327-337.
8.Tran, T., Barlow, B., O’Rear, L., Jarvis, B.,
Li, Z., Dickeson, K., Dupont, W., and Zutter, M. (2011) Loss of the
α2β1 integrin alters human papilloma virus-induced squamous
carcinoma progression in vivo and in vitro, PLoS
One, 6, e26858.
9.Girotti, M. R., Fernandez, M., Lopez, J. A.,
Camafeita, E., Fernandez, E. A., Albar, J. P., Benedetti, L. G.,
Valacco, M. P., Brekken, R. A., Podhajcer, O. L., and Llera, A. S.
(2011) SPARC promotes cathepsin B-mediated melanoma invasiveness
through a collagen I/α2β1 integrin axis, J. Invest.
Dermatol., 131, 2438-2447.
10.Burnier, J. V., Wang, N., Michel, R. P.,
Hassanain, M., Li, S., Lu, Y., Metrakos, P., Antecka, E., Burnier, M.
N., Ponton, A., Gallinger, S., and Brodt, P. (2011) Type IV
collagen-initiated signals provide survival and growth cues required
for liver metastasis, Oncogene, 30, 3766-3783.
11.Li, X., Ishihara, S., Yasuda, M., Nishioka, T.,
Mizutani, T., Ishikawa, M., Kawabata, K., Shirato, H., and Haga, H.
(2013) Lung cancer cells that survive ionizing radiation show increased
integrin α2β1- and EGFR-dependent invasiveness, PLoS
One, 8, e70905.
12.Ramirez, N. E., Zhang, Z., Madamanchi, A., Boyd,
K. L., O’Rear, L. D., Nashabi, A., Li, Z., Dupont, W. D.,
Zijlstra, A., and Zutter, M. M. (2011) The α2β1 integrin is
a metastasis suppressor in mouse models and human cancer, J. Clin.
Invest., 121, 226-237.
13.Ferraro, A., Mourtzoukou, D., Kosmidou, V.,
Avlonitis, S., Kontogeorgos, G., Zografos, G., and Pintzas, A. (2013)
EZH2 is regulated by ERK/AKT and targets integrin alpha2 gene to
control epithelial–mesenchymal transition and anoikis in colon
cancer cells, Int. J. Biochem. Cell Biol., 45,
243-254.
14.Naci, D., Vuori, K., and Aoudjit, F. (2015)
Alpha2beta1 integrin in cancer development and chemoresistance,
Semin. Cancer Biol., 35, 145-153.
15.Guo, Y. S., Zhao, R., Ma, J., Cui, W., Sun, Z.,
Gao, B., He, S., Han, Y. H., Fan, J., Yang, L., Tang, J., and Luo, Z.
J. (2014) βig-h3 promotes human osteosarcoma cells metastasis by
interacting with integrin α2β1 and activating PI3K signaling
pathway, PLoS One, 9, e90220.
16.Naci, D., Azreq, M. A., Chetoui, N., Lauden, L.,
Sigaux, F., Charron, D., Al-Daccak, R., and Aoudjit, F. (2012)
α2β1 integrin promotes chemoresistance against doxorubicin
in cancer cells through extracellular signal-regulated kinase (ERK),
J. Biol. Chem., 287, 17065-17076.
17.Mannava, S., Omilian, A. R., Wawrzyniak, J. A.,
Fink, E. E., Zhuang, D., Miecznikowski, J. C., Marshall, J. R.,
Soengas, M. S., Sears, R. C., Morrison, C. D., and Nikiforov, M. A.
(2012) PP2A-B56a controls oncogene-induced senescence in normal and
tumor human melanocytic cells, Oncogene, 31,
1484-1492.
18.Morozevich, G. E., Kozlova, N. I., Ushakova, N.
A., Preobrazhenskaya, M. E., and Berman, A. E. (2012) Integrin
α5β1 simultaneously controls EGFR-dependent proliferation
and Akt-dependent pro-survival signaling in epidermoid carcinoma cells,
Aging (Albany NY), 4, 368-374.
19.Morozevich, G. E., Kozlova, N. I., Cheglakov, I.
B., Ushakova, N. A., and Berman, A. E. (2009) Integrin α5β1
controls invasion of human breast carcinoma cells by direct and
indirect modulation of MMP-2 collagenase activity, Cell Cycle,
14, 2219-2225.
20.Morozevich, G. E., Kozlova, N. I., Susova, O. Y.,
Karalkin, P. A., and Berman, A. E. (2015) Implication of
α2β1 integrin in anoikis of MCF-7 breast carcinoma cells,
Biochemistry (Moscow), 80, 97-103.
21.Krueger, J. S., Keshamouni, V. G., Atanaskova,
N., and Reddy, K. B. (2001) Temporal and quantitative regulation of
mitogen-activated protein kinase (MAPK) modulates cell motility and
invasion, Oncogene, 20, 4209-4218.
22.Okayama, H. (2012) Cdc6: a trifunctional AAA+
ATPase that plays a central role in controlling the G(1)-S transition
and cell survival, J. Biochem., 152, 297-303.
23.Benetatos, L., Vartholomatos, G., and
Hatzimichael, E. (2014) Polycomb group proteins and MYC: the cancer
connection, Cell. Mol. Life Sci., 71, 257-269.
24.Toulany, M., and Rodemann, H. P. (2016)
Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor
cell responsiveness to radiation, Semin. Cancer Biol.,
35, 180-190.
25.Safdari, Y., Khalili, M., Ebrahimzadeh, M. A.,
Yazdani, Y., and Farajnia, S. (2015) Natural inhibitors of PI3K/AKT
signaling in breast cancer: emphasis on newly-discovered molecular
mechanisms of action, Pharmacol. Res., 93,
1-10.
26.Toker, A., and Yoeli-Lerner, M. (2006) Akt
signaling and cancer: surviving but not moving on, Cancer Res.,
66, 3963-3966.
27.Irie, H. Y., Pearline, R. V., Grueneberg,
D., Hsia, M., Ravichandran, P., Kothari, N., Natesan, S., and
Brugge, J. S. (2005) Distinct roles of Akt1 and Akt2 in regulating cell
migration and epithelial–mesenchymal transition, J. Cell
Biol., 19, 1023-1034.
28.Dyce, O. H., Ziober, A. F., Weber, R. S.,
Miyazaki, K., Khariwala, S. S., Feldman, M., and Ziober, B. L. (2002)
Integrins in head and neck squamous cell carcinoma invasion,
Laryngoscope, 112, 2025-2032.
29.Sottnik, J. L., Daignault-Newton, S., Zhang, X.,
Morrissey, C., Hussain, M. H., Keller, E. T., and Hall, C. L. (2013)
Integrin alpha2beta1 (α2β1) promotes prostate cancer
skeletal metastasis, Clin. Exp. Metastasis, 30, 569-578.
30.Yoshimura, K., Meckel, K. F., Laird, L. S., Chia,
C. Y., Park, J. J., Olino, K. L., Tsunedomi, R., Harada, T., Iizuka,
N., Hazama, S., Kato, Y., Keller, J. W., Thompson, J. M., Chang, F.,
Romer, L. H., Jain, A., Iacobuzio-Donahue, C., Oka, M., Pardoll, D. M.,
and Schulick, R. D. (2009) Integrin alpha2 mediates selective
metastasis to the liver, Cancer Res., 69, 7320-7328.
31.Choi, J. A., Jung, Y. S., Kim, J. Y., Kim, H. M.,
and Lim, I. K. (2016) Inhibition of breast cancer invasion by
TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia
genes, Oncogene, 35, 83-93.
32.Hutchinson, J. N., Jin, J., Cardiff, R. D.,
Woodgett, J. R., and Muller, W. J. (2004) Activation of Akt-1
(PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but
suppresses tumor invasion, Cancer Res., 64,
3171-3178.
33.Arboleda, M. J., Lyons, J. F., Kabbinavar, F. F.,
Bray, M. R., Snow, B. E., Ayala, R., Danino, M., Karlan, B. Y., and
Slamon, D. J. (2003) Overexpression of Akt2/protein kinase Bbeta leads
to up-regulation of beta1 integrins, increased invasion, and metastasis
of human breast and ovarian cancer cells, Cancer Res.,
63, 196-206.
34.Riggio, M., Perrone, M. C., Polo, M. L.,
Rodriguez, M. J., May, M., Abba, M., Lanari, C., and Novaro, V. (2017)
AKT1 and AKT2 isoforms play distinct roles during breast cancer
progression through the regulation of specific downstream proteins,
Sci. Rep., 7; doi: 10.1038/srep44244.
35.Rao, G., Pierobon, M., Kim, I. K., Hsu, W. H.,
Deng, J., Moon, Y.-W., Petricoin, E. F., Zhang, Y. W., Wang, Y., and
Giaccone, G. (2017) Inhibition of AKT1 signaling promotes invasion and
metastasis of non-small cell lung cancer cells with K-ras or EGFR
mutations, Sci. Rep., 7; doi:
10.1038/s41598-017-06128-9.