Protective Effect of Peroxiredoxin 6 Against Toxic Effects of Glucose and Cytokines in Pancreatic RIN-m5F β-Cells
E. G. Novoselova1,a*, O. V. Glushkova1, S. B. Parfenuyk1, M. O. Khrenov1, S. M. Lunin1, T. V. Novoselova1, M. G. Sharapov1, I. A. Shaev1, and V. I. Novoselov1
1Institute of Cell Biophysics, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia* To whom correspondence should be addressed.
Received January 14, 2019; Revised March 18, 2019; Accepted March 18, 2019
Taking into account a special role of pancreatic β-cells in the development of diabetes mellitus, the effects of peroxiredoxin 6 (Prx6) on the viability and functional activity of rat insulinoma RIN-m5F β-cells were studied under diabetes-simulating conditions. For this purpose, the cells were cultured at elevated glucose concentrations or in the presence of pro-inflammatory cytokines (TNF-α and IL-1) known for their special role in the cytotoxic autoimmune response in diabetes. It was found that the increased glucose concentration of 23-43 mM caused death of 20-60% β-cells. Prx6 added to cells significantly reduced the level of reactive oxygen species and protected the RIN-m5F β-cells from hyperglycemia, reducing the death of these cells by several fold. A measurement of insulin secretion by the RIN-m5F β-cells showed a significant stimulatory effect of Prx6 on the insulin-producing activity of pancreatic β-cells. It should be noted that the stimulatory activity of Prx6 was detected during culturing the cells under both normal and unfavorable conditions. The regulation of the NF-κB signaling cascade could be one of the mechanisms of Prx6 action on β-cells, in particular, through activation of RelA/p65 phosphorylation at Ser536.
KEY WORDS: peroxiredoxin 6, hyperglycemia, cytokines, RIN-m5F β-cells, insulin production, signaling cascade NF-κBDOI: 10.1134/S0006297919060063
Abbreviations: carboxy-H2DCFDA, 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (general oxidative stress indicator); GPx, glutathione peroxidase; IL, interleukin; NF-κB, nuclear factor kappa-B; Prx6, peroxiredoxin 6; RIN-m5F cells, rat insulinoma cells; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha.
Disorders in the functioning of pancreatic β-cells are common in
type 1 and 2 diabetes mellitus. Type 1 diabetes mellitus is an
autoimmune disease resulting from destruction of insulin-producing
pancreatic β-cells due to the attack of autoreactive clones of
cytotoxic lymphocytes. The death of the majority of pancreatic
β-cells occurring due to the activation of autoreactive
T-lymphocytes leads to the glucose accumulation in blood, so that type
1 diabetes patients require lifetime use of insulin [1]. Type 2 diabetes mellitus is a severe metabolic
disease characterized by the dysfunction (and not by destruction) of
β-cells and impaired insulin resistance known as glucose toxicity
[2]. Oxidative stress arising during the
development of type 1 and 2 diabetes is believed to be the major
pathophysiological factor of diabetes mellitus [3].
Although treatment of various diabetes-associated pathologies with
antioxidants, mainly, low-molecular-weight natural and synthetic
substances, has been reported [4, 5], it is reasonable to assume that antioxidant
enzymes can be more efficient in their ability to neutralize reactive
oxygen species (ROS) than low-molecular-weight antioxidants. Earlier,
we have shown the therapeutic effect of recombinant peroxiredoxin 6
(Prx6) in various oxidative stress-associated pathologies, including
mechanical and thermal skin traumas, chemical burns of the respiratory
pathways, and hypoxia/reperfusion of the intestine [6-8]. The antioxidative activity
of Prx6 was significantly higher than the activity of known antioxidant
proteins, such as superoxide dismutase (SOD), catalase, and glutathione
peroxidase (GPx). We believe that Prx6 can be an efficient agent for
suppression of oxidative stress in diabetes mellitus. In comparison
with other mammalian tissues, pancreatic β-cells contain lower
levels of antioxidant enzymes (SOD, catalase, and GPx), which makes
them more sensitive to the damaging action of ROS [9]. Because of the insufficiency of endogenous
antioxidant enzymes, it might be interesting to use new proteins that
possess antioxidative activity and are capable of protecting pancreatic
β-cells during the development of diabetes. In this work, we for
the first time studied the effectiveness of Prx6 in the reduction of
damaging effects in rat insulinoma pancreatic cells (RIN-m5F line)
cultured under conditions provoking apoptosis and suppression of the
insulin-producing activity of these cells.
We have shown earlier that diabetes development in animals involves signaling transduction in the immune cells, in which the NF-κB cascade plays a special role [10]. We established that the use of the NF-κB signaling inhibitors significantly lowers the level of immune imbalance in the cells of mice with alloxan-induced diabetes [11]. In the present work, we studied in vitro effects of Prx6 on the NF-κB signaling in β-cells as a pathway involved in the regulation of proinflammatory response during oxidative stress.
MATERIALS AND METHODS
Culturing of RIN-m5F cells. Rat insulinoma RIN-m5F cells (Vertebrate Cell Collection, St. Petersburg, Russia) were grown in cultural flasks in a 1 : 1 mixture of RPMI 1640 and DMEM (Paneco Ltd., Russia) supplemented with 10% fetal calf serum (FCS; Thermo, Great Britain) and antibiotics (100 μg/ml penicillin, 100 µg/ml streptomycin, 50 μg/ml gentamycin) in the presence of low glucose concentrations (8 mM) at 37°C in 5% CO2 atmosphere. The cells were used in the experiments after 3-7 passages. Hyperglycemia was induced by adding glucose in a concentration of 15 to 35 mM; the final glucose concentration was 23-43 mM. Cytokine-induced apoptosis was triggered by adding a mixture of proinflammatory tumor necrosis factor (TNF-α) (30 ng/ml) and interleukin (IL-1β) (15 ng/ml). Prx6 in the concentration of 150 µg/ml was added 30 min before the addition of glucose/cytokines. In each independent experiment, the results were obtained from 9-12 repeats and expressed as mean. To determine the significance of differences between the groups, four independent experiments were performed (n = 4). Cells untreated with glucose, cytokines, or Prx6 were used as the control.
Survival test (cytotoxic test). RIN-m5F cells were cultured in 96-well plates (TPP, Switzerland; 2·104 cells in 100 μl per well) in RPMI 1640 medium (Paneco Ltd.) supplemented with 10% FCS, 2.04 mM L-glutamine (Paneco Ltd.), and 100 μg/ml streptomycin at 37°C in 5% CO2 atmosphere. After culturing for 24 h, 1 μg/ml actinomycin D was added to the formed cell monolayer together with glucose and/or cytokines and Prx6. After another 24 h of culturing, the monolayer was washed three times in PBS and stained with 0.05% crystal violet (Sigma, USA) for 10 min. The wells were then carefully washed with running distilled water, and 100 μl of 1% SDS was added to each well. Absorption of the cell lysates was measured after 10 min at 546 nm with a Titertek Multiscan MCC/340 plate reader (Flow Laboratories, Finland).
Measurement of insulin concentration. RIN-m5F line cells were cultured in 24-well plates (TPP; 1.5·106 cells in 1 ml per well) in RPMI 1640 medium supplemented with 10% FCS, 2.04 mM L-glutamine, and 100 μg/ml streptomycin at 37°C in 5% CO2. After 24 h of culturing, glucose or cytokines and/or Prx6 were added in the above-mentioned concentrations to the formed cell monolayer. After another 24 h of culturing, the cells were disintegrated by three rounds of freezing-thawing, centrifuged (ELMI, Latvia) at 3000g for 10 min, and the supernatant was used the further analysis. Insulin concentration was measured with a commercial Rat Insulin INS ELISA kit (Cloud-Clone Corp., China) as recommended by the manufacturer. Absorption was measured at 450 nm with a Titertek Multiscan MCC/340 plate reader.
Measurement of reactive oxygen species (ROS) using carboxy-H2DCFDA. RIN-m5F line cells were cultured for 24 h in a 96-well plate (2.5·104 cells in 100 µl per well) in DMEM and then washed with PBS. The cells were then incubated with carboxy-H2DCFDA (freshly prepared in sterile DMSO; Invitrogen, USA) in a final concentration of 2.5 μM in the medium supplemented with 2% depleted FCS in the darkness for 1 h; then Prx6 and glucose were added, and the cells were incubated for another 1 h. The cells incubated in the absence of Prx6 and glucose were used as a control. The fluorescence at 530 nm was measured with an Infinite 200 plate-reader (Tecan, Austria) (excitation at 480 nm) as described earlier [12].
SDS-PAGE and immunoblotting. To prepare samples for electrophoresis, 2·106 cells were disintegrated by sonication with an UZDN-2T ultrasonic disintegrator (Russia) on ice at continuous stirring for 2 min. Proteins were precipitated with acetone; 2× solubilizing buffer (50 mM Tris-HCl, 2% SDS, 25% (v/v) glycerol, 5% β-mercaptoethanol and 0.1% bromophenol blue, pH 6.8) was then added to the samples at a 1 : 1 ratio. The samples were boiled for 5 min. The final protein concentration in the samples was 1 mg/ml (as determined by Bradford method [13]); 10 μl of each sample was loaded on a gel. After the transfer of fractionated proteins onto the membranes, the membranes were probed for the proteins of interest using the following antibodies: rabbit antibodies to phospho-NF-κB (Ser276) (Santa Cruz, USA), rabbit antibodies to phospho-NF-κB (Ser536), and rabbit antibodies to caspase-3 (Cell Signaling, USA).
The proteins were detected with an ECL system (GE Healthcare, Sweden), and the developed blots were photographed using a TFX-35.WL trans-illuminator (Vilber Lourmat, France). The amounts of the proteins were determined densitometrically using the Qapa program (v. 3.7). Three independent experiments were performed (using the cells from different passages) for each protein. The obtained data were normalized to the corresponding load control (GAPDH) and expressed in relative units.
Statistical analysis was performed using the Student’s t-test.
RESULTS
The effect of Prx6 on the viability of pancreatic β-cells was studied in vitro in insulinoma RIN-m5F cells. It is known that the proliferative and regenerative abilities of pancreatic β-cells are closely associated with the amount of released insulin; there is an inverse correlation between insulin secretion and glucose concentration in the blood [14]. RIN-m5F β-cells were used as a model of diabetes mellitus, and the viability of these cells was studied under unfavorable conditions, such as increased glucose concentrations or the presence of proinflammatory cytokines. We also analyzed the levels of insulin secretion and apoptosis, activity of the NF-κB signaling cascade, and effectiveness of the protective influence of Prx6. The antioxidant activity of Prx6 in the cells was tested using carboxy-H2DCFDA. The obtained results indicated that Prx6 significantly decreased the level of ROS in RIN-m5F cells cultured in the presence of 33 mM glucose (table).
Effect of Prx6 on the ROS levels in RIN-m5F cells in the presence of
increased glucose concentration

Note: The cells were incubated for 1 h in the presence of the
fluorescent dye carboxy-H2DCFDA and 33 mM glucose and/or
Prx6 (150 µg/ml). Each value presents a mean for green
fluorescence intensity from 9-12 repeats (calculated as % of the
control).
* Significant difference with the control, p < 0.01.
# Significant difference with the group
“glucose”, p < 0.05.
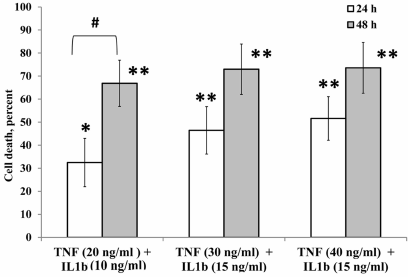
Effect of proinflammatory cytokines on cell survival. Figure 1 shows the data on the survival of β-cells in the presence of the proinflammatory cytokines TNF-α and IL-1β. Incubation with a mixture of these two proinflammatory cytokines for 24 and 48 h induced the death of pancreatic β-cells. The cytotoxic effect of the proinflammatory cytokines was more pronounced with the increase in the exposure, as well as in the concentration of the added cytokines. Thus, culturing the cells for 24 h at the maximal concentration of the cytokines (40 ng for TNF-α and 15 ng for IL-1β) caused the death of 50% RIN-m5F cells; when the exposure time was increased, more than 60% cells died under the same conditions.
Fig. 1. Death of RIN-m5F β-cells cultured for 24 and 48 h in the presence of cytokines. * Significant difference with the control, p < 0.05; ** significant difference with the control, p < 0.01; # significant difference between 24 and 48 h.
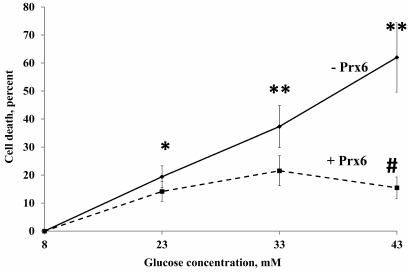
Effect of Prx6 on the survival of β-cells under hyperglycemic conditions. In the following series of experiments, we studied the survival of RIN-m5F β-cells in the presence of increased glucose concentrations in the culture medium and the effect of Prx6 addition on the cell survival (Fig. 2). An increase in the glucose concentration to 23-43 mM caused the death of 20-60% β-cells. At the same time, addition of Prx6 reliably protected the cells against hyperglycemia by decreasing 5-fold the number of dead cells (at the glucose concentration of 43 mM). These results are very important because the observed direct protection of pancreatic β-cells by Prx6 from the hyperglycemia-induced death allowed us to suggest that this antioxidant enzyme could be also effective under in vivo conditions. Survival of β-cells under unfavorable hyperglycemic conditions in the presence of Prx6 rose a question about the functional activity of these cells, whose main role is insulin secretion.
Fig. 2. Protective effect of Prx6 on the viability of RIN-m5F cells cultured for 24 h in the high-glucose medium. * Significant difference with the control, p < 0.05; ** significant difference with the control, p < 0.01; # significant difference with the cells cultured without Prx6 in the presence of 43 mM glucose, p < 0.01.
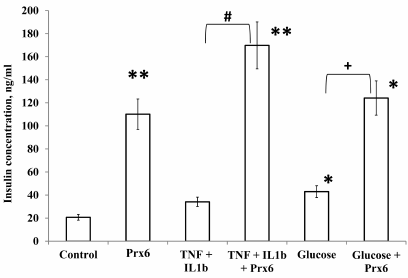
Effect of Prx6 on insulin secretion of by the RIN-m5F β-cells. The results shown in Fig. 3 demonstrate a pronounced stimulatory effect of Prx6 on the insulin-producing activity of the pancreatic β-cells. It is interesting that the stimulatory effect of Prx6 was detected when β-cells were cultivated under normal conditions and under unfavorable conditions leading to the cell death. In fact, the addition of Prx6 to the culture medium rapidly enhanced the insulin-producing activity of the cells both in the presence of cytokines and under hyperglycemic conditions.
Fig. 3. Production of insulin by RIN-m5F cells cultured for 24 h in the presence of high glucose concentrations (33 mM), cytokines (40 ng/ml TNF-α and 15 ng/ml IL-1β), Prx6, and combinations of these compounds. * Significant difference with the control, p < 0.01; ** significant difference with the control, p < 0.05; # significant difference with the “cytokines” group, p < 0.01; + significant difference with the “glucose” group, p < 0.01.
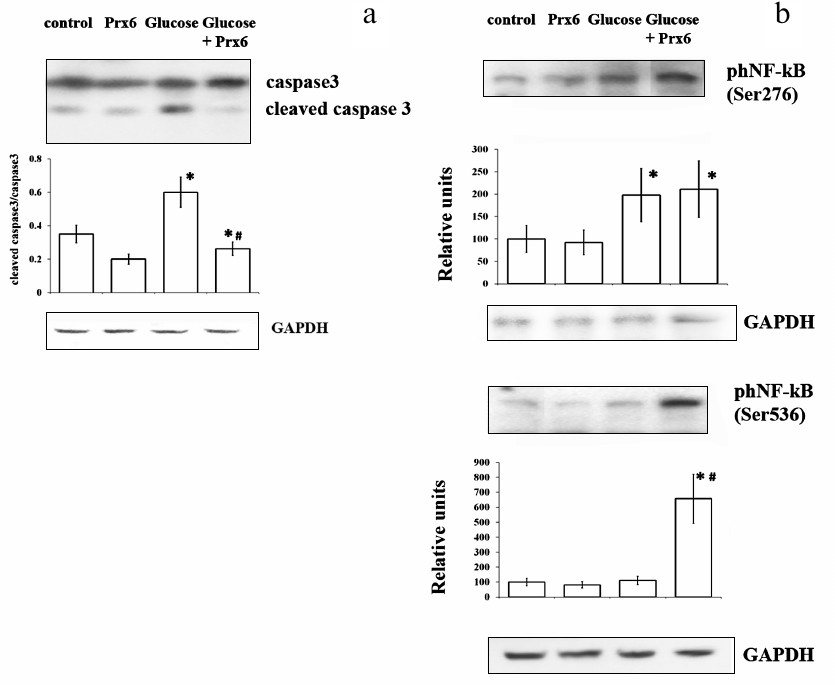
Effect of Prx6 on apoptosis and activity of NF-κB cascade in RIN-m5F cells. The apoptosis levels in RIN-m5F β-cells were assessed by measuring the activated/inactivated caspase-3 ratio (Fig. 4a). It was shown that elevated glucose concentrations (33 mM) significantly activated apoptosis, whereas Prx6 completely abolished the effect of hyperglycemia on the cells by normalizing the ratio between the activated and inactivated forms of caspase-3.
Fig. 4. Effect of Prx6 on apoptosis (a) and NF-κB signaling cascade activity (b) in RIN-m5F β-cells cultured in the presence of 33 mM glucose for 24 h. Representative Western blots are shown; the histograms show the mean values from three independent experiments. a) Histograms under the blot indicate the ratio between two caspase forms; * significant difference with the control, p < 0.05; # significant difference between the “glucose” and “Prx6 + glucose” groups, p < 0.01. b) Histograms under the blots show the amount of protein (as measured by densitometry; relative units); * significant difference with the control, p < 0.05; # significant difference between the “glucose” and “Prx6 + glucose” groups. GAPDH was used as a load control.
Transcription factor NF-κB is a key component of the cell response to stress, damage, and inflammation [15]. In the cytoplasm, NF-κB is present as a p65/p50 heterodimer which is maintained in its inactive state by IκB proteins. In response to various factors, including oxidative stress, NF-κB is activated through phosphorylation of IκB and release of p65, which leads to the NF-κB translocation to the nucleus and triggers expression of genes regulating cytokine production [16]. It is known that the transcriptional specificity of p65/NF-κB is determined by its post-translational modifications. The most important phosphorylation sites in p65 are Ser276 and Ser536. Ser536 is phosphorylated by kinases IKK, TANK (TBK1), and RSK1; Ser276 is phosphorylated mainly by protein kinase A (PKA) in the cytoplasm and MSK1 kinase in the nucleus [17]. The post-translational modification of p65 via phosphorylation of Ser536 has been described virtually in all types of unspecific cell response, and its biological role is similar to the function of phospho-Ser276-p65. While activation at Ser276 increases the half-life of p65 [18], phosphorylation of Ser536 with the involvement of IKK is associated with the increased proteasomal degradation of this transcription factor in activated macrophages [19]. Thus, phosphorylation of p65 at Ser276 is likely associated with the cell survival, whereas phosphorylation at Ser536 can lead to the cell death via apoptosis. The activity of the NF-κB signaling cascade was assessed from phosphorylation of RelA/p65 at two serine residues (Ser276 and Ser536) (Fig. 4b). We showed that culturing the cells in the presence of 33 mM glucose increased 2-fold the phosphorylation level of RelA/p65 at Ser276 but did not change the extent of phosphorylation at Ser536. Interestingly, Prx6 addition to the cells resulted in more pronounced phosphorylation of RelA/p65 at Ser536, while phosphorylation of NF-κB at Ser276 did not change. It is important to note that in the absence of increased glucose levels, Prx6 addition also significantly increased the NF-κB cascade activity without influencing the level of RIN-m5F cell apoptosis.
Therefore, Prx6 exhibited a pronounced in vitro protective effect on β-cells by increasing their survival and insulin-producing activity in the presence of increased glucose concentrations. It has been proven that the effect of Prx6 on β-cells is realized through activation of the NF-κB signaling cascade via increase in the extent of RelA/p65 phosphorylation at Ser536.
DISCUSSION
Dysfunction of pancreatic β-cells is a key factor in the emergence and progress of diabetes mellitus. Impaired functioning of β-cell contributes to the decrease in insulin secretion and subsequent accelerated development of the disease. In this work, we for the first time used the antioxidant enzyme Prx6 for protection of RIN-m5F β-cells. We were able to demonstrate the efficiency of Prx6 in both reducing the level of β-cell apoptosis under hyperglycemic conditions and restoring their ability to secrete insulin.
In this connection, it seemed important to study the effects of Prx6 on the insulin secretion by β-cells, as well as on the survival of these cells under unfavorable conditions caused by the presence of cytokines or increased glucose concentrations. In this study, RIN-m5F cells grown in the high-glucose medium were less viable than the cells grown at low glucose concentrations, which is in accordance with the previously obtained data. However, by using Prx6, we were able to significantly promote the survival of cells cultured under hyperglycemic conditions. These new data suggest that the use of Prx6 might be a promising approach for reducing pathological consequences of diabetes mellitus.
It is known that proinflammatory cytokines (TNF-α and IL-1β) are important components in the pathogenesis of the 1 type diabetes [20], β-cells being the targets for these cytokines. The destructive action of IL-1β is realized through inhibition of the MafA transcription factor and insulin gene transcription [21]. Production and secretion of this cytokine is induced by high glucose levels and promote the death of β-cells [22]. Here, we have shown that the presence of TNF-α and IL-1β decreases the survival of RIN-m5F cells, while Prx6 addition to the culture medium protects the cells against the toxic effect of the cytokines by increasing their ability to produce insulin. Thus, it has been established that in the presence of Prx6, pancreatic β-cells cultured under unfavorable conditions (for instance, at high glucose levels or in presence of proinflammatory cytokines) retain their ability for increased insulin secretion. We believe that this observation is the most important result of the present work.
A question arises about the mechanism of Prx6 action on the insulin secretion by β-cells cultured under normal conditions. In fact, Prx6 stimulates insulin production independently of the presence of glucose or cytokines. Other authors have studied the influence of the peroral GPx mimetic (antioxidant enzyme) on the changes in the insulin blood levels in young rats and found that in the antioxidant-treated animals, the level of insulin significantly increased by the 14th week [23]. The authors believed that this was due to the upregulation of two transcriptional factors – Pdx1 and MafA – which are important for insulin synthesis. It is very likely that Prx6 is also involved in the regulation of insulin production, but this hypothesis should be verified experimentally.
Various pathologies associated with oxidative stress in animals have been studied, and it was established that the mechanism of Prx6 protective action is due to at least two factors: the peroxidase activity of the enzyme and the regulatory function of Prx6 not associated with its peroxidase activity, as it was shown in our review [24].
Based on the results of previous studies, NF-κB activation can trigger either pro- or anti-apoptotic cascades [25]. Pancreatic β-cells are known to be a target of the autoimmune response provoked by proinflammatory cytokines that activate NF-κB and promote its translocation to the cell nucleus [26]. These cytokines modify expression of hundreds of genes, resulting in the dysfunction of β-cells and their death [27]. Some of the cytokine-induced genes are regulated via NF-κB activation and might be the targets for NF-κB [28].
Other data show that the activated NF-κB signaling cascade can play a protective role in β-cells. Thus, a model of transgenic diabetic mice was obtained, in which NF-κB in pancreatic β-cells was inhibited [29]. It was shown in this work that a long-term blockade of NF-κB in newborn animals during the pancreas development downregulated expression of the key genes of the insulin secretion pathway and reduced the total number of endocrine cells in the pancreas. This does not contradict the data obtained by Kim et al. [30] who demonstrated that NF-κB prevents cell death and development of the type 1 diabetes in transgenic NOD mice, indicating that NF-κB could play an anti-apoptotic role in β-cells of these mice and potentially prevent diabetes development.
Here, we demonstrated that addition of Prx6 to RIN-m5F β-cells significantly increases activity of the NF-κB cascade. We conclude that on the background of increased viability and insulin-secreting activity of β-cells, NF-κB displays the anti-apoptotic activity.
Funding. The work was supported by the Russian Foundation for Basic Research (projects nos. 18-04-00091 and 19-04-00080-a) and Russian Academy of Sciences Presidium Program 1.18 “Molecular and Cell Biology and Post-genomic Techniques”.
Acknowledgements. In the work, we used the equipment of the Pushchino Scientific Center Collective Use Center (an Infinite 200 plate reader; Tecan, Austria).
Conflict of interest. The authors declare no conflict of interest.
Ethical norm compliance. This article does not contain description of studies with the involvement of humans or animal subjects.
REFERENCES
1.Hober, D., and Sane, F. (2010) Enteroviral
pathogenesis of type 1 diabetes, Discov. Med., 10,
151-160.
2.Kaneto, H., Katakami, N., Kawamori, D., Miyatsuka,
T., Sakamoto, K., Matsuoka, T. A., Matsuhisa, M., and Yamasaki, Y.
(2007) Involvement of oxidative stress in the pathogenesis of diabetes,
Antioxid. Redox Signal., 9, 355-366.
3.Rains, J. L., and Jain, S. K. (2011) Oxidative
stress, insulin signaling, and diabetes, Free Radic. Biol. Med.,
50, 567-575, doi: 10.1016/j.freeradbiomed.2010.12.006.
4.Wojnar, W., Zych, M., and Kaczmarczyk-Sedlak, I.
(2018) Antioxidative effect of flavonoid naringenin in the lenses of
type 1 diabetic rats, Biomed. Pharmacother., 108,
974-984, doi: 10.1016/j.biopha.2018.09.092.
5.Czerwinska, M. E., Gasinska, E., Lesniak, A.,
Krawczyk, P., Kiss, A. K., Naruszewicz, M., and Bujalska-Zadrozny, M.
(2018) Inhibitory effect of Ligustrum vulgare leaf extract on
the development of neuropathic pain in a streptozotocin-induced rat
model of diabetes, Phytomedicine, 49, 75-82, doi:
10.1016/j.phymed.2018.06.006.
6.Gordeeva, A. E., Sharapov, M. G., Tikhonova, I. V.,
Chemeris, N. K., Fesenko, E. E., Novoselov, V. I., and Temnov, A. A.
(2017) Vascular pathology of ischemia/reperfusion injury of rat small
intestine, Cells Tissues Organs, 203, 353-364, doi:
10.1159/000455830.
7.Sharapov, M. G., Goncharov, R. G., Gordeeva, A. E.,
Novoselov, V. I., Antonova, O. A., Tikhaze, A. K., and Lankin, V. Z.
(2016) Enzymatic antioxidant system of endotheliocytes, Dokl.
Biochem. Biophys., 471, 410-412, doi:
10.1134/S1607672916060090.
8.Karaduleva, E. V., Mubarakshina, E. K., Sharapov,
M. G., Volkova, A. E., Pimenov, O. Y., Ravin, V. K., Kokoz, Y. M., and
Novoselov, V. I. (2016) Cardioprotective effect of modified
peroxiredoxins in retrograde perfusion of isolated rat heart under
conditions of oxidative stress, Bull. Exp. Biol. Med.,
160, 639-642, doi: 10.1007/s10517-016-3237-1.
9.Kaneto, H., Kajimoto, Y., Miyagawa, J., Matsuoka,
T., Fujitani, Y., Umayahara, Y., Hanafusa, T., Matsuzawa, Y., Yamasaki,
Y., and Hori, M. (1999) Beneficial effects of antioxidants in diabetes
– possible protection of pancreatic β-cells against glucose
toxicity, Diabetes, 48, 2398-2406.
10.Novoselova, E. G., Khrenov, M. O., Parfenyuk, S.
B., Novoselova, T. V., Lunin, S. M., and Fesenko, E. E. (2014) The
NF-κB, IRF3 and SAPK/JNK signaling cascades of animal immune
cells and their role in the progress of type 1 diabetes mellitus,
Dokl. Biol. Sci., 457, 255-257, doi:
10.1134/S0012496614040073.
11.Novoselova, E. G., Glushkova, O. V., Lunin, S.
M., Khrenov, M. O., Novoselova, T. V., Parfenyuk, S. B., and Fesenko,
E. E. (2016) Signaling, stress response and apoptosis in pre-diabetes
and diabetes: restoring immune balance in mice with alloxan-induced
type 1 diabetes mellitus, Intern. Immunopharm., 31,
24-31, doi: 10.1016/j.intimp.2015.11.007.
12.Wu, D., and Yotnda, P. (2011) Production and
detection of reactive oxygen species (ROS) in cancers, J. Vis.
Exp., 57, e3357, doi: 10.3791/3357.
13.Bradford, M. M. (1976) A rapid and sensitive
method for the quantitation of microgram quantities of protein
utilizing the principle of protein–dye binding, Anal.
Biochem., 72, 248-254.
14.Weir, G. C., and Bonner-Weir, S. (2004) Five
stages of evolving β-cell dysfunction during progression to
diabetes, Diabetes, 53, Suppl. 3, S16-S21.
15.Hayden, M. S., and Ghosh, S. (2008) Shared
principles in NF-κB signaling, Cell, 132, 344-362,
doi: 10.1016/j.cell.2008.01.020.
16.Bubici, C., Papa, S., Dean, K., and Franzoso, G.
(2006) Mutual cross-talk between reactive oxygen species and nuclear
factor-kappa B: molecular basis and biological significance,
Oncogen, 25, 6731-6748, doi: 10.1038/sj.onc.1209936.
17.Vermeulen, L., De Wilde, G., Van Damme, P.,
Vanden Berghe, W., and Haegeman, G. (2003) Transcriptional activation
of the NF-κB p65 subunit by mitogen- and stress-activated protein
kinase-1 (MSK1), EMBO J., 22, 1313-1324, doi:
10.1093/emboj/cdg139.
18.Nihira, K., Ando, Y., Yamaguchi, T., Kagami, Y.,
Miki, Y., and Yoshida, K. (2010) Pim-1 controls NF-kappaB signaling by
stabilizing RelA/p65, Cell Death Differ., 17, 689-698,
doi: 10.1038/cdd.2009.174.
19.Lawrence, T., Bebien, M., Liu, G. Y., Nizet, V.,
and Karin, M. (2005) IKKalpha limits macrophage NF-kappaB activation
and contributes to the resolution of inflammation, Nature,
434, 1138-1143, doi: 10.1038/nature03491.
20.Imai, Y., Dobrian, A. D., Morris, M. A., and
Nadler, J. L. (2013) Islet inflammation: a unifying target for diabetes
treatment? Trends Endocrinol. Metab., 24, 351-360, doi:
10.1016/j.tem.2013.01.007.
21.Oetjen, E., Blume, R., Cierny, I., Schlag, C.,
Kutschenko, A., Kratzner, R., Stein, R., and Knepel, W. (2007)
Inhibition of MafA transcriptional activity and human insulin gene
transcription by interleukin-1beta and mitogen activated protein kinase
kinase kinase in pancreatic islet beta cells, Diabetologia,
50, 1678-1687, doi: 10.1007/s00125-007-0712-2.
22.Maedler, K., Sergeev, P., Ris, F., Oberholzer,
J., Joller-Jemelka, H. I., Spinas, G. A., Kaiser, N., Halban, P. A.,
and Donath, M. Y. (2002) Glucose-induced beta cell production of
IL-1beta contributes to glucotoxicity in human pancreatic islets, J.
Clin. Invest., 110, 851-860, doi: 10.1172/JCI15318.
23.Mahadevan, J., Parazzoli, S., Oseid, E., Hertzel,
A. V., Bernlohr, D. A., Vallerie, S. N., Liu, C. Q., Lopez, M., Harmon,
J. S., and Robertson, R. P. (2013) Ebselen treatment prevents islet
apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves
β-cell mass and function in ZDF rats, Diabetes, 62,
3582-3588, doi: 10.2337/db13-0357.
24.Sharapov, M. G., Novoselov, V. I., and Gudkov, S.
V. (2019) Radioprotective role of peroxiredoxin 6, Antioxidants
(Basel), 8, E15, doi: 10.3390/antiox8010015.
25.Barkett, M., and Gilmore, T. D. (1999) Control of
apoptosis by Rel/NF-kappa B transcription factors, Oncogene,
18, 6910-6924.
26.Cardozo, A. K., Heimberg, H., Heremans, Y.,
Leeman, R., Kutlu, B., Kruhoffer, M., Orntoft, T., and Eizirik, D. L.
(2001) A comprehensive analysis of cytokine-induced and nuclear
factor-kappa B dependent genes in primary rat pancreatic beta-cells,
J. Biol. Chem., 276, 879-886, doi:
10.1074/jbc.M108658200.
27.Eizirik, D. L., and Mandrup-Poulsen, T. (2001) A
choice of death – the signal-transduction of immune-mediated
beta-cell apoptosis, Diabetologia, 44, 2115-2133, doi:
10.1007/s001250100021.
28.Larsen, P. M., Fey, S. J., Larsen, M. R.,
Nawrocki, A., Andersen, H. U., Kahler, H., Heilmann, C., Voss, M. C.,
Roepstorff, P., Pociot, F., Karlsen, A. E., and Nerup, J. (2001)
Proteome analysis of interleukin-1beta-induced changes in protein
expression in rat islets of Langerhans, Diabetes, 50,
1056-1063.
29.Norlin, S., Ahlgren, U., and Edlund, H. (2005)
Nuclear factor-kappa B activity in beta-cells is required for
glucose-stimulated insulin secretion, Diabetes, 54,
125-132.
30.Kim, S., Millet, I., Kim, H. S., Kim, J. Y., Han,
M. S., Lee, M. K., Kim, K. W., Sherwin, R. S., Karin, M., and Lee, M.
S. (2007) NF-kappa B prevents beta cell death and autoimmune diabetes
in NOD mice, Proc. Natl. Acad. Sci. USA, 104, 1913-1918,
doi: 10.1073/pnas.0610690104.