Polymorphism of the IL-1β, TNF, IL-1RA and IL-4 Cytokine Genes Significantly Increases the Risk of Preterm Birth
V. S. Belousova1,a*, O. A. Svitich2, E. V. Timokhina1,b*, A. N. Strizhakov1, and I. M. Bogomazova1
1Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation, 119991 Moscow, Russia2Mechnikov Research Institute of Vaccines and Sera, 105064 Moscow, Russia
* To whom correspondence should be addressed.
Received November 19, 2018; Revised April 11, 2019; Accepted May 21, 2019
Preterm birth is not only medical, but also a social problem. The global goal of medicine is prevention of preterm labor and identification of risk factors leading to preterm birth. The objective of our study was to find the association between polymorphic markers in the cytokine IL-1β, TNF-α, IL-1Ra, and IL-4 genes and development of preterm labor. The prospective study was conducted in 108 pregnant women with the risk of preterm birth. The main group consisted of 66 women whose pregnancy ended with preterm delivery despite the ongoing therapy. The comparison group included 42 women with the full-term delivery. The dominant T allele of the cytokine IL-1β gene polymorphism rs1143634 (3953C→T) was 7.6 times more common in women with preterm delivery vs. the comparison group (36.4 and 4.8%, respectively; RR, 1.802; 95% CI, 1.420-2.288; p < 0.05); its homozygous form was detected only in women with preterm delivery at the very early gestation age (less than 26 weeks). The dominant proinflammatory allele 2R of the IL-1 receptor antagonist gene (IL-1Ra) was 1.5 times more common in women with preterm delivery than in the comparison group (63.6 and 42.8%, respectively; RR, 1.400; 95% CI, 1.009-1.943; p < 0.05), which makes the 2R allele the risk factor for preterm birth. The 2R/2R and 2R/4R genotypes led to a very early and early preterm delivery, respectively. The combination of three or four proinflammatory genotypes was detected only in women with a very early preterm delivery, which confirms that the combination of several proinflammatory genotypes is an extremely unfavorable factor for the full-term pregnancy. Identification of genetic polymorphisms in the interleukin genes at the periconceptional stage will help to prevent the risk of preterm delivery, which will reduce the incidence of preterm births, as well as perinatal morbidity and mortality.
KEY WORDS: preterm labor, cytokines, IL-1β, IL-1Ra, IL-4, IL-4, TNF-α, interleukins, cytokine gene polymorphismDOI: 10.1134/S0006297919090062
Abbreviations: CI, confidence interval; IL, interleukin; IL-1Ra, IL-1 receptor antagonist; NK cell, natural killer cell; PD, preterm delivery; RR, relative risk; SNP, single nucleotide polymorphism; TNF-α, tumor necrosis factor alpha; VNTR, variable number of tandem repeats.
Preterm delivery (PD) is a medical and social issue. The PD rate ranges
from 6.5% (Germany, France, Italy) and 7.8% (Great Britain, Russia) to
12% (USA). Annually, 15 million preterm neonates are born worldwide,
100,000 of them – in Russia. Moreover, preterm babies contribute
most to the perinatal mortality and account for 75% of stillbirths and
deaths in the first week of life [1]. Severe
disabilities and serious developmental disorders among preterm
survivors reach up to 15.4 and 14.8%, respectively [2].
PD reis caused by premature uterine contraction leading to the cervix softening and dilation ending up with the premature expulsion of the fetus. It is virtually impossible to stop PD once it started. Therefore, it is essential to prevent PD by identification of risk factors, prophylactic measures, and timely therapeutic intervention.
The prevalent etiological factor triggering both preterm and full-term delivery is inflammation development of in the uteroplacental complex. Secreted pro-inflammatory cytokines found in the cervical mucus, myometrium, placenta, amniotic fluid, blood plasma, and umbilical cord blood play a crucial role in the delivery onset [1, 3-5].
In some pregnant women (7-10%), the inflammatory cascade might be activated prematurely before the end of the full-term pregnancy. Why does it happen? It is commonly believed now that that the key role in triggering belongs to the innate immune secretory factors (pro- and anti-inflammatory cytokines, antimicrobial peptides, etc.), receptors (Toll-like receptors, etc.), and innate immune cells. In pregnant women, the balance between adaptive immune factors is shifted toward the humoral arm. Cytokines regulate the labor onset, cervical dilation, and rupture of membranes via activity of the pro-inflammatory arm. Infectious agents (viruses, bacteria, fungi, protozoa) are sensed by the Toll-like receptors (transmembrane glycoproteins located on the surface of different types of cells), as well as intracellularly. This triggers an activation signal that eliciting production of pro-inflammatory cytokines, such as interleukins, tumor necrosis factor-α (TNF-α), etc. In the cervix, pro-inflammatory cytokines activate production of matrix metalloproteinases belonging to the zinc/calcium-dependent endopeptidase family that degrade extracellular matrix components in the connective tissue. The activity of metalloproteinases alters the structural composition of the cervix by lowering the content of proteoglycans and truncating and disrupting the collagen bundles, which results in cervix softening and subsequent PD [3].
However, in some women, infectious agents are not identified despite high levels of pro-inflammatory cytokines, which are presumably caused by other factors (e.g., genetically determined predisposition to upregulated synthesis of pro-inflammatory cytokines related to the polymorphism of the corresponding genes) [3, 6, 7]. It is known that TNF-α is the major pro-inflammatory cytokine released by macrophages, neutrophils, and natural killer (NK) cells. It was found that in physiological pregnancy, the serum levels of TNF-α are low, but may be sharply elevated in the case of developing infectious processes, particularly, urogenital infection. Normal pregnancy requires low TNF-α levels; TNF-α interacts with cognate surface receptors on the trophoblast cells, thereby protecting them from the activity of maternal cytotoxic T cell clones. Excessive production of TNF-α may negatively affect the pregnancy. Sidelnikova and Sukhikh demonstrated that the serum levels of TNF-α in pregnant women with the risk of PD in the 3rd trimester are ~9 times higher than in women with normal pregnancy [5]. Early in pregnancy, high amounts of TNF-α suppress migration of trophoblasts and initiate cell apoptosis in the placenta [4].
Pro-inflammatory cytokines IL-1β and IL-1 receptor antagonist (IL-1Ra) are also involved in PD development. IL-1β is a multifunctional cytokine that displays a broad range of activities and plays a crucial role in the development and regulation of innate and adaptive immunity via cross-talking with TNF-α in immune response [6]. IL-1β mediates the emergence of systemic inflammatory response and exhibits biological effects similar to those of TNF-α by activating leukocytes, endothelial cells, and complement system, as well as production of acute phase proteins [7]. IL-1β acts simultaneously on several cell types by upregulating production of cyclooxygenase-2 and prostaglandin E2, the two most efficient agents in cervical dilation. Pro-inflammatory cytokines also cause changes in the myometrium. Hertelendy et al. showed that IL-1β and TNF-α elicit myometrial production of arachidonic acid, activate phospholipid metabolism, and promote prostaglandin production [8]. Such effects of IL-1β on the myometrial cells are similar to the action of oxytocin, which together with prostaglandin E2 can elevate the calcium levels in the myometrial cells required for the uterine contraction.
IL-1Ra regulates expression and functional activity of the key pro-inflammatory cytokine IL-1β. Imbalanced production of IL-1β and IL-1Ra results in prolonged inflammatory response associated with tissue and organ damage [9].
In contrast to the upregulated production of pro-inflammatory cytokines associated with PD, other cytokines (e.g., the anti-inflammatory cytokine IL-4) can exert the counteracting protective effect on pregnancy. Thus, elevated amounts of IL-4 during pregnancy provide maternal immunosuppressive effect against developing fetoplacental complex [4, 10-12]. IL-4 restricts production of pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-12, and TNF-α by macrophages, inhibits generation of reactive oxygen and nitrogen species, activates macrophages, and induces proliferation of NK cells [13]. Suppression of the pro-inflammatory arm ensures progression of the full-term pregnancy.
The risk of PD is associated with the shift in the cytokine profile toward the pro-inflammatory one with the minimal content of anti-inflammatory cytokines.
Currently, the markers of PD are extensively searched for. However, in most of such studies, the methods used for measuring cytokines as PD biomarkers are characterized by low sensitivity and specificity and often provide controversial data, which requires searching for a combination of predictive factors.
Due to the development of molecular and genetics approaches, PD is now considered as a maternal genotype-linked pregnancy complication. Currently, the studies of PD are mostly focused on the genetically determined features underlying the risk of PD. The majority of these studies examine the pro-inflammatory cytokine genes, such as IL-1B, IL-4, and TNF. The most frequent alterations in the genes (including cytokine genes) are single nucleotide polymorphisms (SNPs). These genetic variations have a great impact on shaping the individual’s anti-microbial immune response; they can also affect the course of pregnancy and labor onset. The majority of identified SNPs in cytokine gene are within the regulatory motifs that directly affect gene transcriptional activity and, as a result, cytokine levels in the blood serum [14]. The association between PD and SNPs in the TNF gene has been most studied. For instance, the A→G substitution at position –308 in the TNF promoter region (TNF –308) results in the upregulated TNF-α production associated with the aberrant course of inflammatory response. The presence of this allele elevates the risk of PD 2-fold, regardless of its homo- or heterozygous state.
Some gene polymorphisms decrease the risk of developing PD in the case of infection.
The mechanisms underlying PD prevention or promotion by certain SNPs remain obscure. For instance, they can involve upregulation of protein production, activation of gene promoters, or downregulation of transcription of inhibitors controlling certain genes. Currently, the role of cytokine gene polymorphisms in the predisposition to PD is still debated. It should be noted that the populational aspect also contributes to the allele and genotype prevalence of certain cytokine gene polymorphisms. In view of this, searching for association between cytokine gene polymorphisms and PD is of high priority in obstetrics.
Here, we attempted to identify an association between the polymorphisms in the cytokine genes IL-1B, TNF, IL-1RA, and IL-4 (single genes and their combinations) and the development of early (weeks 28-32) and very early (before week 28) PD, which might be used for the PD prediction and prevention.
MATERIALS AND METHODS
Clinical groups. The prospective study was conducted in 108 pregnant women with a risk of developing PD. The following inclusion criteria were applied for the enrollment: singlet pregnancy at weeks 23-36; pulling pain in the lower abdomen; shortening of the cervix (<25 mm) according to the ultrasound examination; and informed consent to be enrolled in the study. The following exclusion criteria were applied: blood-tinged vaginal discharge; pregnancy due to assisted reproductive technologies; severe somatic pathologies and decompensated chronic health conditions; chromosomal aberrations and fetal congenital abnormalities; infection; complicated pregnancy (preeclampsia and related complications, placental insufficiency).
The main group consisted of 66 women, whose pregnancy ended with PD despite the therapy. The comparison group included 42 pregnant females who had full-term delivery.
All pregnant women enrolled in the study underwent clinical and laboratory examination, including collection of medical history, general clinical and special obstetric examination, vaginal examination assessing the state of the cervix; ultrasound cervicometry; examination of fetal functional state and uterine contractile activity patterns.
The following polymorphisms with the autosomal dominant inheritance in four cytokine genes were examined as potential PD predictors: rs1143634 (+3953C→T) in IL-1B; rs1800629 (G→A) in TNF; VNTR in IL-1RA; and VNTR (intron 3) in IL-4. Genomic DNA was isolated from the buccal swabs by the phenol–chloroform method [15].
IL-1Ra is a protein product of the IL-1RA gene. Polymorphic VNTR variants of the IL-1RA and IL-4 (intron 3) genes were identified by PCR with subsequent detection of the amplified DNA fragments after electrophoretic separation in 6% polyacrylamide gel, staining with ethidium bromide, and visualization in the UV light. The gels were photographed with a digital video camera 221S (Vatec, Japan) [16, 17].
Polymorphic variants for the IL-1B gene (rs1143634, +3953C→T) and TNF gene (rs1800629, G→A) were determined by PCR in a Mastercycler amplifier (Eppendorf, Austria) followed by endonuclease restriction analysis of the amplified products (SibEnzyme, Russia). The digestion products were analyzed by 7% PAGE, staining with ethidium bromide, and visualization in the UV light. The gels were photographed with a digital video camera 221S (Vatec) [18].
Statistical analysis was performed using common statistical methods with the software packages BioStat and Excel. Qualitative data (allele and genotype frequencies for the polymorphic markers) were processed using the non-parametric χ2 and Fisher’s exact tests. Statistical data processing for the polymorphic markers was mainly performed using the χ2 test for analysis of the four-field tables to assess the prevalence of alleles, genotypes, and haplotypes in the main and comparison groups. The significance level was set at p < 0.05; the relative risk (RR) and the confidence interval (CI) were also calculated [18].
RESULTS
Here, we assessed the allele prevalence for the rs1143634 polymorphism (+3953C→T) of the cytokine IL-1B gene. The dominant T allele was found in 36.4% females with PD, whereas in the comparison group, it was found only in 4.8% women (p < 0.05; RR, 1.802; 95% CI, 1.420-2.288). The TT genotype was identified in 9.1% women with PD (p < 0.01), who were also characterized by very early PD at week 26 of gestation.
The CT heterozygous genotype was found in 27.3% females with PD and only in 4.8% women in the comparison group (p < 0.01; RR, 1.65; 95% CI, 1.298-2.098). PD in the women with the CT heterozygote occurred at weeks 27-32 of gestation.
The CC genotype was found in 63.6% women with PD at weeks 33-36 of gestation vs. 95.2% women with the full-term delivery (p < 0.01; RR, 0.555; 95% CI, 0.437-0.704) (Table 1).
Table 1. Prevalence of cytokine gene
polymorphisms in women with PD (main group) and full-term birth
(comparison group)
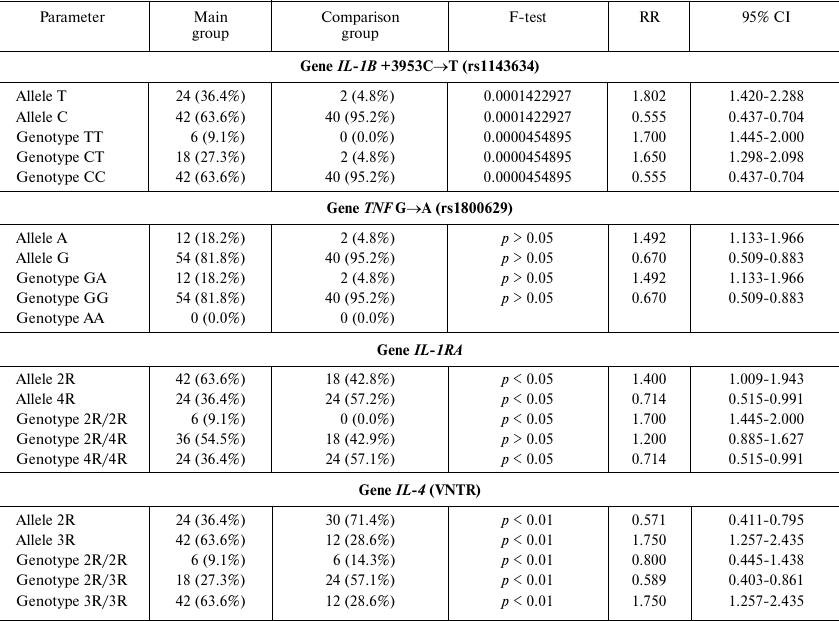
No carriers homozygous by the dominant pro-inflammatory allele A of the rs1800629 (G→A) polymorphism in the TNF gene were found. The pro-inflammatory GA genotype was identified in 18.2% pregnant women in the main group, but only in 4.8% females in the comparison group (main group: p > 0.05; RR, 1.492; 95% CI, 1.133-1.966). In carriers of the pro-inflammatory allele A, PD occurred at weeks 26-30, whereas in women with the GG genotype, delivery took place after week 31 of gestation. In the comparison group, the homozygous GG genotype was identified in 95.2% women (p > 0.05; RR, 0.670; 95% CI, 0.509-0.883) (Table 1).
Examination of the VNTR polymorphism of the interleukin 1 receptor antagonist gene IL-1RA revealed that its dominant pro-inflammatory allele 2R was 1.5-fold more frequent in women with PD vs. the comparison group: 63.6 vs. 42.8%, respectively (p < 0.05; RR, 1.400; 95% CI, 1.009-1.943). Importantly, the 2R/2R genotype was detected solely in females with PD (9.1%; p < 0.05; RR, 1.714; 95% CI, 1.445-2.000). Women of the main group also tended to carry the 2R/4R genotype at a higher frequency that women in the comparison group (54.5 vs. 42.9%, respectively; p > 0.05).
Women with the homozygous 2R/2R or heterozygous 2R/4R genotypes had PD before week 26 or at weeks 26-31 of gestation, respectively (Table 1).
Furthermore, it was found that the gene IL-4 can carry the dominant pro-inflammatory allele 2R. Molecular genetic analysis revealed that the prevalence of the 2R allele was 2 times lower in women with PD than in the comparison group (36.4 vs. 71.4%, respectively; p < 0.01; RR, 0.571; 95% CI, 0.411-0.795). The prevalence of the 2R/2R in the main group was 1.6 times lower than in the comparison group (9.1 vs. 14.3%; RR, 0.8; 95% CI, 0.445-1.438; p < 0.01), while the prevalence of the 2R/3R genotype was 2.1 times lower than in the comparison group (27.3 vs. 57.1%; RR, 0.589; 95% CI, 0.403-0.861; p < 0.01).
The pro-inflammatory 3R/3R genotype was found 2 times more often in females with PD than in the comparison group (63.6 vs. 28.6%; RR, 1.750; 95% CI, 1.257-2.435; p < 0.01). Women with the 3R/3R genotype had PD at weeks 23-30; women with the 2R/3R and 2R/2R genotypes had PD after week 32 of gestation.
Next, we examined the association between a combination of several pro-inflammatory genotypes and pregnancy outcome (Table 2). No significant differences in frequency and onset of PD was observed in carriers of either one or two pro-inflammatory alleles. However, simultaneous presence of three or four pro-inflammatory alleles was observed only in women in the main group: combination of three alleles – in 9.1% women with PD (p < 0.05; RR, 1.700; 95% CI, 1.445-2.000); combination of four alleles – in 18.2% women with PD (p < 0.01; RR, 1.778; 95% CI, 1.490-2.121). Moreover, the onset of PD in all carriers of three or four pro-inflammatory alleles occurred at the very early stages of gestation (Table 2).
Table 2. Distribution of combined
pro-inflammatory genotypes and pregnancy outcomes
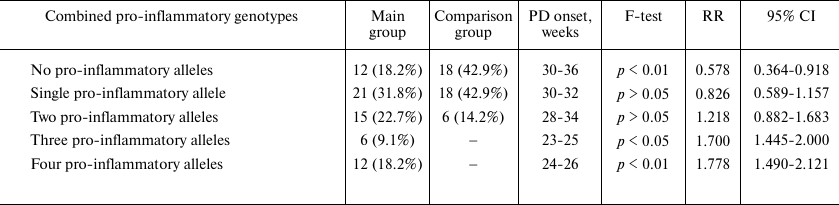
In addition, we found that the GA genotype of the rs1800629 (G→A) polymorphism of the TNF gene was always combined with the pro-inflammatory genotypes of the rs1143634 polymorphism (+3953C→T) of the IL-1B gene, VNTR polymorphism of IL-1RA gene, and VNTR polymorphism of the IL-4 (intron 3) gene, resulting in the very early PD (before week 27 of gestation). Moreover, the TT genotype of the rs1143634 polymorphism (+3953C→T) of the IL-1B gene was always combined with the pro-inflammatory genotypes of the VNTR polymorphism of IL-1RA gene, and VNTR polymorphism of the IL-4 (intron 3) gene, resulting in early PD at weeks 23-25 of gestation.
The heterozygous variant CT of the rs1143634 polymorphism (+3953C→T) of the IL-1B gene was associated in 66.7% the with the pro-inflammatory genotypes of the rs1800629 (G→A) polymorphism of the TNF gene and VNTR polymorphism of the IL-1RA gene, resulting in very early PD. In the case of heterozygous CT genotype of the rs1143634 polymorphism (+3953C→T) of the IL-1B gene alone, without other polymorphisms, PD was observed at weeks 32-33 of gestation.
DISCUSSION
Currently, the polymorphism of cytokine genes is extensively studied. The role of pro-inflammatory cytokines in developing PD has been commonly accepted by now. Multiple studies have demonstrated that the levels of pro-inflammatory cytokines are upregulated in the amniotic fluid, placenta, myometrium, and umbilical cord blood in PD patients [20, 21].
However, the reasons underlying the elevated production of pro-inflammatory cytokines have not been entirely clarified. It is suggested that one of causes of the increased cytokine production might be linked to the cytokine gene polymorphism. Yilmaz et al. examined the polymorphism at position +3954 in the IL-1B gene and found that the TT genotype dominated in women with PD, whereas the preterm neonates delivered by these women displayed significantly higher occurrence of the TT variant both in the IL-1A and IL-1B genes [21]. In our study, we observed that the prevalence of the dominant T allele in the rs1143634 polymorphism (+3953C→T) of the IL-1B gene was 7.6 times higher in women with PD than in the comparison group (36.4 vs. 4.8%, respectively; RR, 1.802; 95% CI, 1.420-2.288, p < 0.05); the homozygous TT variant was observed solely in patients with PD, who had delivery at very early stage (before 26 weeks of gestation). Therefore, the presence of the pro-inflammatory T allele may be suggested as a risk factor of developing PD, whereas the TT or CT genotypes are associated with in very early or early PD.
The TNF gene has several polymorphisms (–237 G>A, –308 G>A, –307 G>A, –857 C>T, –863 C>A, –1032 T>C, –238 G>A, –851 G>A, –323 G>A, +691 G>A, and –376 G>A) that were found to be associated with PD in numerous studies [1, 21]. It was shown that the AA genotype at position –308 and the GA and AA genotypes at position –238 of the TNF gene are more common in women with PD [22, 23]. However, the TNF gene polymorphisms rs1799964, rs1799724, and rs1800629 were not linked to PD [24]. In our study, we observed that the prevalence of the pro-inflammatory allele A in rs1800629 polymorphism of the TNF gene was 3.8 times higher in women with PD than in the comparison group (18.2 vs. 4.8%, respectively; RR, 1.492; 95% CI, 1.133-1.966; p > 0.05), and even its heterozygous variant resulted in early PD. However, the lack of significant difference between the main and comparison groups in both cases does not allow to consider the pro-inflammatory A allele of the rs1800629 polymorphism (G→Α) in the TNF gene as a risk factor of early and very early PD.
Although Bitner and Kalinka revealed no significant differences in the prevalence of the pro-inflammatory 2R allele of the IL-1RA gene in women with PD and full-term delivery [17]; the majority of studies have demonstrated that the 2R allele is reliably linked to the premature termination of pregnancy [25-27]. We found that the dominant pro-inflammatory 2R allele of the gene IL-1RA (VNTR) is 1.5-fold more frequent in women with PD than in the comparison group (63.6 vs. 42.8%, respectively; RR, 1.400; 95% CI, 1.009-1.943; p < 0.05). Therefore, these data allow to suggest with assurance that the dominant 2R allele is a risk factor for developing PD. Moreover, the 2R/2R genotype vs. the 2R/4R genotype results in very early vs. early PD.
The anti-inflammatory cytokine IL-4 exhibits a protective effect. In particular, it suppresses activity of ПЕPЕBОДЧИК: противоречиеmacrophages in the extravascular matrix, which results in the inhibition of inflammatory response [1]. However, it was previously shown that the 509C/C genotype of the IL-4 gene increases the risk of developing PD [28]. Zhu et al. demonstrated that the polymorphism within the control locus of the IL-4 gene is associated with development of spontaneous PD [1]. The 2R/2R or 2R/3R variants of the IL-4 gene prevent PD development. Individuals with the 3R/3R genotype do not produce IL-4, which results in PD. Here, we found the 3R/3R genotype at a significantly higher frequency in women with PD than in the comparison group (63.6 vs. 28.6%, respectively; RR, 1.750; 95% CI, 1.257-2.435; p < 0.01). Hence, the 3R/3R genotype of the IL-4 (VNTR) gene can considered as a risk factor of very early and early PD.
The combination of pro-inflammatory genotypes of several cytokine genes is also a risk factor of PD, because it results in upregulated production of the corresponding cytokines. We found such combinations of three or four pro-inflammatory cytokine alleles solely in women with very early PD, thereby confirming the assumption that a combination of several pro-inflammatory genotypes represents a highly unfavorable factor for the full-time pregnancy despite the therapeutic interventions aimed to prevent PD.
Hence, assessment of the frequency of allelic variants of cytokine genes in women with previously recorded PD has a great prognostic value for predicting an outcome of subsequent pregnancies. Revealing genetic features of the immune status of women before pregnancy will be helpful for timely implementation of prophylactic measures and PD prevention, which will lower the number of PDs, as well as perinatal morbidity and mortality.
Acknowledgements. We are thankful to the staff of the Yudin City Clinical Hospital, Moscow Department of Health, for assistance and support and to the Laboratory of Genetics, Mechnikov Research Institute of Vaccines and Sera, for performing genetic studies.
Conflict of interest. The authors declare no conflict of interest.
Compliance with ethical standards. All procedures with human subjects performed in the study comply with the ethics regulations approved by the Institute and/or National Ethics Committee, as well as the 1964 Declaration of Helsinki and its amendments or comparable ethical standards. All human subjects enrolled in the study provided voluntary informed consent.
REFERENCES
1.Zhu, Q., Sun, J., and Chen, Y. (2014) Preterm birth
and single nucleotide polymorphisms in cytokine genes, Transl.
Pediatr., 3, 120-134, doi:
10.3978/j.issn.2224-4336.2014.03.02.
2.Payne, A. H., Hintz, S. R., Hibbs, A. M., Walsh, M.
C., Vohr, B. R., Bann, C. M., and Wilson-Costello, D. E. (2013)
Low-gestational-age neonates with low-grade
periventricular-intraventricular hemorrhage, JAMA Pediatr.,
167, 451-459, doi: 10.1001/jamapediatrics.2013.866.
3.Strizhakov, A. N., Belousova, V. S., and Svitich,
O. A. (2016) Clinical importance of Toll-like receptors in pathogenesis
of preterm delivery, Vopr. Ginekol. Akusher. Perinatal.,
15, 35-41, doi: 10.20953/1726-1678-2016-1-35-40.
4.Amirova, Zh. S. (2006) A cytokine network in
pregnant women with persistent and relapsing threatened miscarriage,
Vest. Nov. Med. Tekhnol., 4, 66-67.
5.Sidel’nikova, V. M., and Sukhikh, G. T.
(2010) Habitual Miscarriage: Guidelines for Medical
Practitioners [in Russian], MIAS, Moscow.
6.Belfer, I., Buzas, B., Hipp, H., Doevendans, P. A.,
ten Cate, H., Prins, M. H., and Biesma, D. H. (2004) Haplotype
structure of inflammatory cytokines genes (IL1B, IL6 and
TNF/LTA) in US Caucasians and African Americans, Genes
Immun., 6, 505-512, doi: 10.1038/sj.gene.6364118.
7.Imangulova, M. M., Bikmaeva, A. R., and
Khusnutdinova, E. K. (2005) Interleukin 1 gene cluster polymorphism in
patients with pulmonary tuberculosis, Tsitokin. Vospalen.,
4, 36-41.
8.Hertelendy, F., Molnar, M., and Romero, R. (2002)
Interferon γ antagonizes interleukin-1β-induced
cyclooxygenase-2 expression and prostaglandin E2 production in human
myometrial cells, J. Soc. Gynecol. Investig., 9,
215-219.
9.Settin, A., Abdel-Hady, H., El-Baz, R., and Saber,
I. (2007) Gene polymorphisms of TNF-α−308,
IL-10−1082, IL-6−174, and
IL-1RaVNTR related to susceptibility and severity of
rheumatic heart disease, Pediatr. Cardiol., 28, 363-371,
doi: 10.1007/s00246-006-0002-7.
10.Tsybul’skaya, O. V., Zharkin, N. A., and
Burova, N. A. (2012) Current aspects in preventing early miscarriage,
Lekarstv. Vestnik, 6, 3-7.
11.Casart, Y., Tarrazzi, K., and Camejo, M. (2007)
Serum levels of interleukin-6, interleukin-1β and human chorionic
gonadotropin in pre-eclamptic and normal pregnancy, Gynecol.
Endocrinol., 23, 300-320, doi:
10.1080/09513590701327638.
12.Salamonsen, L. A. (2007) Cytokines and chemokines
during human embrioimplantation: roles in implantation and early
placentation, Semin. Reprod. Med., 25, 437-444, doi:
10.1055/s-2007-991041.
13.Simbirtsev, A. S., and Gromova, A. Yu. (2005)
Functional gene polymorphism for cytokines as regulatory molecules in
inflammation, Tsitokin. Vospalen., 4, 3-10.
14.Maniatis, T., Frich, E., and Sembruk, J. (1984)
Genetic Engineering Methods. Molecular Cloning [Russian
translation], Mir, Moscow.
15.Kamenarska, Z., Dzhebir, G., Hristova, M., Savov,
A., Vinkov, A., Kaneva, R., Mitev, V., and Dourmishev, L. (2014) IL-1RN
VNTR polymorphism in adult dermatomyositis and systemic lupus
erythematosus, Dermatol. Res. Pract., 5, 101-106,
doi: 10.1155/2014/953597.
16.Makhlouf, M. M., and Abd Elhamid, S. M. (2014)
Expression of IL4 (VNTR intron 3) and IL10 (–627) genes
polymorphisms in childhood immune thrombocytopenic purpura, Lab.
Med., 45, 211-219, doi: 10.1309/LMB0QC5T1RXTTRZQ.
17.Bitner, A., and Kalinka, J. (2010) IL-1β,
IL-6 promoter, TNF-α promoter and IL-1RA gene polymorphisms and
the risk of preterm delivery due to preterm premature rupture of
membranes in a population of Polish women, J. Arch. Med. Sci.,
6, 552-557, doi: 10.5114/aoms.2010.14467.
18.Stenton, G. (1999) Medical and Biological
Statistics [Russian translation], Praktika, Moscow.
19.Salminen, A., Paananen, R., Vuolteenaho, R.,
Metsola, J., Ojaniemi, M., Autio-Harmainen, H., and Hallman, M. (2008)
Maternal endotoxin-induced preterm birth in mice: fetal responses in
toll-like receptors, collectins, and cytokines, Pediatr. Res.,
63, 280-286, doi: 10.1203/PDR.
20.Makarov, O. V., Koval’chuk, L. V., and
Gankovskaya, L. V. (2007) Habitual Miscarriage, Infection, Innate
Immunity [in Russian], Geotar-Media, Moscow.
21.Yilmaz, Y., Verdi, H., Taneri, A., Yazici, A. C.,
Ecevit, A. N., Karakas, N. M., Tarcan, A., Haberal, A., Ozbek, N., and
Atac, F. B. (2012) Maternal-fetal proinflammatory cytokine gene
polymorphism and preterm birth, DNA Cell Biol., 31,
92-97, doi: 10.1089/dna.2010.1169.
22.Moura, E., Mattar, R., de Souza, E., Torloni, M.
R., Goncalves-Primo, A., and Daher, S. (2009) Inflammatory cytokine
gene polymorphisms and spontaneous preterm birth, J. Reprod.
Immunol., 80, 115-121, doi: 10.1016/j.jri.2008.11.007.
23.Harper, M., Zheng, S. L., Thom, E., Klebanoff, M.
A., Thorp, J., Sorokin, Y., Varner, M. W., Iams, J. D., Mercer,
B. M., Rouse, D. J., Ramin, S. M., and Anderson, G. D. (2011)
Cytokine gene polymorphisms and length of gestation, Obstet.
Gynecol., 117, 125-130, doi:
10.1097/AOG.0b013e318202b2ef.
24.Jones, N. M., Holzman, C., Friderici, K. H.,
Jernigan, K., Chung, H., Wirth, J., and Fisher, R. (2010) Interplay of
cytokine polymorphisms and bacterial vaginosis in the etiology of
preterm delivery, J. Reprod. Immunol., 87, 82-89, doi:
10.1016/j.jri.2010.06.158.
25.Heinzmann, A., Mailaparambil, B., Mingirulli, N.,
and Krueger, M. (2009) Association of interleukin-13/-4 and toll-like
receptor 10 with preterm births, Neonatology, 96,
175-181, doi: 10.4103/ijmr.IJMR_1624_14.
26.Genc, M. R., Gerber, S., Nesin, M., and Witkin,
S. S. (2002) Polymorphism in the interleukin-1 gene complex and
spontaneous preterm delivery, Am. J. Obstet. Gynecol.,
187, 157-163.
27.Kalish, R. B., Vardhana, S., Gupta, M., Chasen,
S. T., Perni, S. C., and Witkin, S. S. (2003) Interleukin-1 receptor
antagonist gene polymorphism and multifetal pregnancy outcome, Am.
J. Obstet. Gynecol., 189, 911-914, doi:
10.5114/aoms.2010.14467.
28.Murtha, A. P., Nieves, A., Hauser, E. R., Swamy,
G. K., Yonish, B. A., Sinclair, T. R., and Heine, R. P. (2006)
Association of maternal IL-1 receptor antagonist intron 2 gene
polymorphism and preterm birth, Am. J. Obstet. Gynecol.,
195, 1249-1253, doi: 10.1016/j.ajog.2006.09.002.