REVIEW: Biochemical Regulation of Regenerative Processes by Growth Factors and Cytokines: Basic Mechanisms and Relevance for Regenerative Medicine#
P. I. Makarevich1,2,a*, A. Yu. Efimenko1,2, and V. A. Tkachuk1,2,3
1Lomonosov Moscow State University, Institute for Regenerative Medicine, Medical Research and Education Center, 119991 Moscow, Russia2Lomonosov Moscow State University, Faculty of Fundamental Medicine, 119991 Moscow, Russia
3Institute of Experimental Cardiology, National Medical Research Center of Cardiology, 121552 Moscow, Russia
# The article is dedicated to the 80th anniversary of the Department of Biochemistry, Faculty of Biology, Lomonosov Moscow State University (see Volume 84, Issue 11, 2019).
* To whom correspondence should be addressed.
Received June 15, 2019; Revised September 30, 2019; Accepted October 16, 2019
Regenerative medicine that had emerged as a scientific and medical discipline at end of 20th century uses cultured cells and tissue-engineered structures for transplantation into human body to restore lost or damaged organs. However, practical achievements in this field are far from the promising results obtained in laboratory experiments. Searching for new directions has made apparent that successful solution of practical problems is impossible without understanding the fundamental principles of the regulation of development, renewal, and regeneration of human tissues. These aspects have been extensively investigated by cell biologists, physiologists, and biochemists working in a specific research area often referred to as regenerative biology. It is known that during regeneration, growth factors, cytokines, and hormones act beyond the regulation of individual cell functions, but rather activate specific receptor systems and control pivotal tissue repair processes, including cell proliferation and differentiation. These events require numerous coordinated stimuli and, therefore, are practically irreproducible using single proteins or low-molecular-weight compounds, i.e., cannot be directed by applying classical pharmacological approaches. Our review summarizes current concepts on the regulatory mechanisms of renewal and regeneration of human tissues with special attention to certain general biological and evolutionary aspects. We focus on the biochemical regulatory mechanisms of regeneration, in particular, the role of growth factors and cytokines and their receptor systems. In a separate section, we discussed practical approaches for activating regeneration using small molecules and stem cell secretome containing a broad repertoire of growth factors, cytokines, peptides, and extracellular vesicles.
KEY WORDS: regenerative medicine, stem cell, growth factor, cytokine, intracellular signaling, receptor tyrosine kinaseDOI: 10.1134/S0006297920010022
Abbreviations: EGF, epidermal growth factor; FGF, fibroblast growth factor; GF, growth factor; GT, gene therapy; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; MSC, mesenchymal stromal cells; PDGF, platelet-derived growth factor; RTK, receptor tyrosine kinase; SC, stem cells; VEGF, vascular endothelial growth factor.
In living systems, regeneration occurs at all levels – molecules
are synthesized and destroyed; damaged tissues are rejuvenated and
restored; some cells die, but others emerge [1].
Continuous regeneration ensures physiological rejuvenation that
maintains the correspondence between a body and its structure adopted
during the evolution, whereas renewal at the molecular level is
necessary for sustained intra-tissue interactions and regulation of
ongoing processes therein.
Humans are inferior to many other multicellular organisms by their potential for reparative (i.e., post-injury) regeneration; nevertheless, the processes of tissue renewal proceed in the human body over the entire lifespan at a remarkable scale. Almost all cells exist for a certain period of time culminated by the programmed cell death. Dead cells are replaced for a limited number of times with newly emerging cells of the same type, which underlies tissue homeostasis (stability of cell composition) [2, 3]. In adult human organism, ~1% mature erythrocytes are replaced every day, i.e., 1011 red blood cells are destroyed, and the same number of these cells emerge every 24 h; 2 to 3 million new erythrocytes exit each second from the bone marrow [4]. Continuous cell renewal occurs at varying rates in the skin, adipose tissue, all parenchymal and hollow organs, heart, and nervous system. The death and emergence of new cells might be compared to the direct and reverse reactions with constants changing at different timepoints of life, as well as in cases of injury or disease [5].
Here, we should mention the classic work Principles of Regeneration by Richard J. Goss, in which he brilliantly noticed: “If there were no regeneration there could be no life. If everything regenerated there would be no death. All organisms exist between these two extremes” [1].
Another successful analogy belongs to David L. Stocum: “As individuals, we use regeneration to locally reverse the second law of thermodynamics for a short time, a struggle we ultimately lose, but one that we win as a species over a longer span of time through reproduction” [6].
As noted above, mammals are inferior in their potential to regenerate compared to other vertebrates, e.g., tailed amphibians or fish that can grow limbs or even internal organs many times.
The first question that emerged after looking at such examples was: “How do they do it”? The answer to this question was proposed by the fundamental studies on animal regeneration that have been carried out from the middle of XIX century. The idea that deciphering regeneration mechanisms in species capable of it might help to understand how similar process could be triggered in humans seemed quite logical [7] and led to the discovery of cellular, molecular, and genetic principles underlying this phenomenon by using appropriate living objects. Investigating vertebrates with remarkable regeneration potential has answered the question of “how”, but not another critical question: “Why do some species lack such capacities”? [8].
The answer to the second question might be found in comparative studies of two closely related species, one of which is able to regenerate limbs or organs (species A), whereas the other one heals injuries by forming stumps or scars (species B). Such comparison might not only allow to understand the events driving regeneration in species A, but also to elucidate what blocks regeneration in species B, e.g., what regulatory system or signaling axis had been lost or dramatically changed after the two taxa diverged.
Such approach was partially implemented in the studies on wound healing in mammalian embryos capable of full regeneration, while adult individuals of the same species form scars at the site of injury [9, 10]. These studies have had a great impact on our understanding of the regeneration potential in mammals, although they have suffered from the common drawbacks. In particular, a growing organism at the embryonic and fetal stages is characterized by an incomplete tissue and organ development, specific hematopoiesis, and immature immune system. It is surrounded by the water environment and exists in the hypoxic state [11, 12].
In utero, fetal cells and DNA are protected from the damaging effects of reactive oxygen species and radiation. Considering the scale of organismal remodeling, birth, to some extent, can be compared to event when first animals moved from water to land, except that newborns spend only few first days of life for adaptation instead of millions of years. Because of the exposure to atmospheric oxygen, an organism after birth experiences a tremendous stress while adapting to a new level of tissue oxygenation [13, 14]. Within the first week of life, this causes an avalanche of epigenetic modifications that affect expression of hundreds of genes encoding various proteins and small regulatory RNAs [15, 16], which is considered as one of the reasons for significantly reduced regenerative potential in humans and land mammals [16]. Based on this notion, reasonable doubts have been put forward as whether comparing regeneration efficiency in embryos and adult individuals is relevant and can produce breakthrough data.
REPARATIVE REGENERATION IN HUMAN, ITS NATURE AND MAJOR CELL-BASED
MECHANISMS
The origin of regenerative capacity in species has always been envisioned from two evolutionary perspectives. The first one considers limb and organ regeneration as an adaptive feature, which some species have been able to develop. Retaining this feature requires selective pressure, e.g., attacks by natural predators or injury by multiple external factors. However, a chance for survival of an individual that had lost its body part is extremely low. Even if we accept that the capacity to grow back fins in fish or tail in lizards has emerged as an adaptation after attacks by natural predators, it is more difficult to justify the adaptive origin of regeneration of internal organs (heart, pancreas, or liver). The damage to these organs almost inevitably results in death that would hardly provide an opportunity for retaining any new trait [17]. Instead, the more realistic and broadly accepted viewpoint is that regeneration is a residual trait, i.e., a specific response established during intrauterine development that had been evolutionarily lost in mammals but remained in amphibians and reptiles [18].
The discoveries in the regenerative biology in the late XX century have laid the foundation for a new field called regenerative medicine. However, further progression of regenerative medicine took place in isolation from the classical research directions in biology, which resulted in its shift toward accelerated translational studies without full understanding of fundamental basics behind regeneration and organism development. The majority of methods for promoting regeneration in humans are based on the transplantation of cultured cells or tissue-engineered constructs [19]. Over the last decades, the focus of these studies has been mostly on applied and technological solutions without proper attention to the fundamental principles behind the functioning of systems controlling human regeneration.
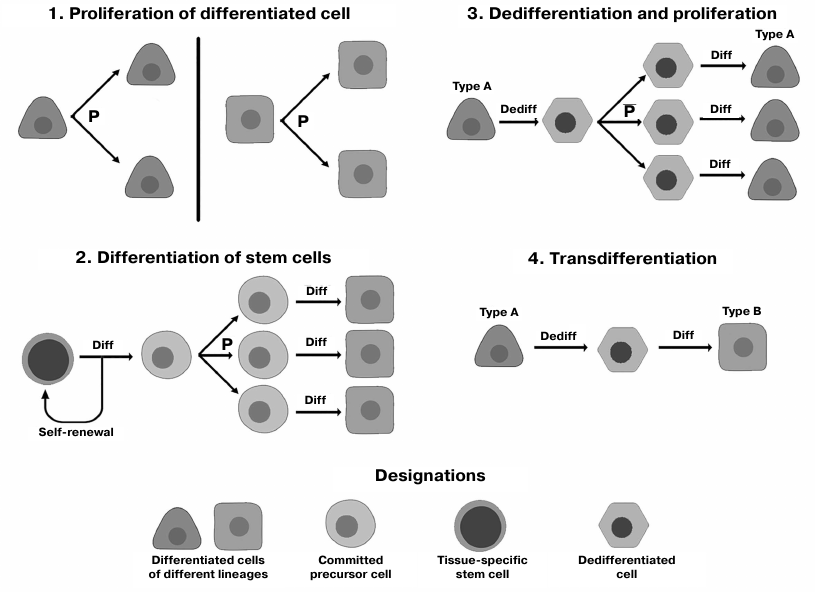
Reparative regeneration in humans occurs via a number of diverse mechanisms (Fig. 1).
Fig. 1. Major mechanisms of human reparative regeneration (see the text for more detail); P, proliferation; Dediff, dedifferentiation; Diff, differentiation.
Differentiation of stem cells (SCs). A pool of tissue-specific (resident) SCs capable of self-renewal (division without losing undifferentiated state) and differentiation into specialized lineage-restricted cell types is preserved and maintained postnatally in the majority of body tissues [20]. Together with mesenchymal stromal cells (MSCs), SCs ensure tissue homeostasis and physiological tissue renewal, as well as play a key role in regeneration after injuries [21]. Moreover, MSCs maintain SCs [22] by creating a specific environment (niche) around them [21].
Proliferation of differentiated cells. Cell division has been considered as the main mechanism of tissue regeneration since formulation of the concepts of the unified cell theory [23]. Later, it was found that the proliferative potential of actively renewing tissues (mucosa, liver, skin) may be insufficient for maintaining tissue structural stability because of the Hayflick limit and age-related decline in cell proliferation [24, 25]. Moreover, multiple proliferation rounds are accompanied by accumulation of errors and changes in the cell differentiation state, which underlies development of regenerative diseases and cancer [26]. Therefore, cell proliferation plays a pivotal role in regeneration, but from a long-term perspective, its opportunities are limited.
Transitory dedifferentiation. In low-plasticity tissues, some of the new cells during regeneration emerge via transitory dedifferentiation. Mature maternal cell gives rise to two daughter cells bearing similar phenotype and functional potential by progressing via the dedifferentiated state with a high degree of plasticity. Damaged cells undergo epigenetic modifications that allow their temporary return to a less specialized state, in which they can divide with the formation of cells that undergo redifferentiation and replace dead cells [27]. This mechanism was described for the regeneration in some human exocrine glands, e.g., salivary gland and pancreas [28, 29].
Direct transdifferentiation is conversion of mature cell of one type into another cell type without returning into the low-differentiated state associated with significant changes in the cell potency. The reprogramming occurs upon activation of specific transcription factors usually expressed during embryogenesis (Oct-4, Klf-4, Nanog, etc.), as well as in response to regulatory (micro- and long noncoding) RNAs [30]. It allows the cell to skip the pluripotency state (that poses a threat due to the possibility of carcinogenesis) and to convert into another specialized cell type [31]. Regeneration via direct transdifferentiation occurs in humans after injury in the islets of Langerhans [32, 33] and hepatic bile ducts [34, 35].
Regeneration and repair processes proceed for significant periods of time and are regulated by hormones, growth factors (GFs), cytokines, various classes of RNAs, etc. [36-38]. Below, we discuss regulatory systems affecting tissue regeneration and renewal and some of their features critical from the biochemical and medical viewpoints.
REGULATION OF RENEWAL AND REGENERATION IN HUMANS
Neuroendocrine regulation of renewal and regeneration. The functions of all body cells are governed by the neuroendocrine system that involves regulation by neurotransmitters and hormones differing in the timing and duration of the response. The nervous system is characterized by a rapid, almost instant, response induced by neurotransmitters released into the synaptic cleft (acetylcholine, serotonin, norepinephrine, etc.). Their cognate receptors are ionotropic ligand-gated transmembrane ion channels [39, 40]. Activation of these channels causes changes in the membrane polarization, and the response is triggered and quenched in a fraction of a second, thereby providing an extremely rapid regulatory circuit underlying multiple reflexes (including those necessary for the survival in the emergency settings) [41].
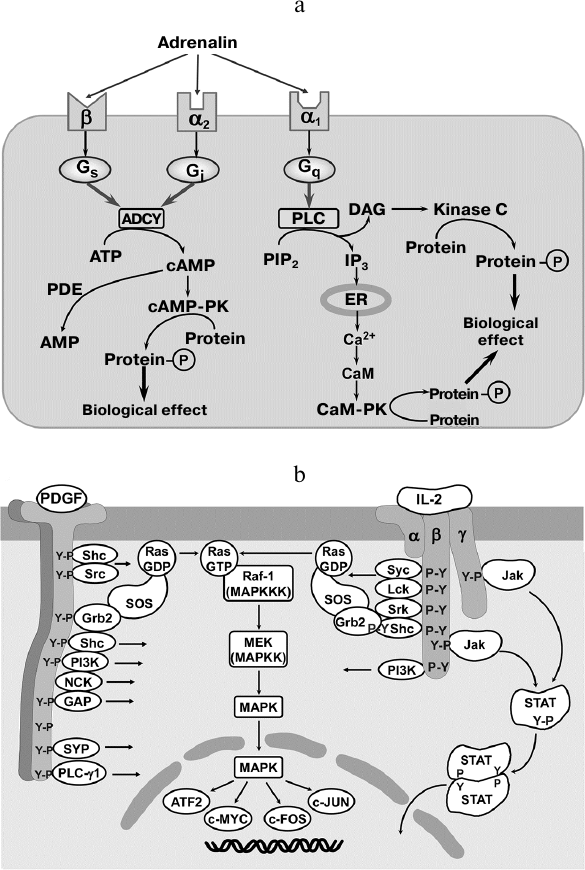
In contrast, the effects of hormones last for longer time intervals (minutes, hours) and are mediated by the membrane-bound and intracellular receptors located in the cytosol, organelle membranes, and nucleus. Hormonal regulation is not an “on/off” system, but rather resembles a rheostat mechanism guided by a number of different cell membrane receptors recognizing the same hormone (Fig. 2a). For instance, the adrenergic system includes simultaneously expressed transmembrane β- and α-adrenergic receptors coupled to the Gs- and Gi-proteins that regulate adenylate kinase activity and intracellular Ca2+ ion concentration in opposite directions [42].
Fig. 2. Signaling pathways triggered by hormones, GFs, and interleukins. a) A hormone may activate different signaling cascades depending on the receptor type. An agonist (adrenalin) acts via one out of the three specific receptors that transduce signals through different G-proteins, adenylate kinase system, and phosphoinositide metabolism. ADCY, adenylate cyclase; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; cAMP, cyclic adenosine monophosphate; IP3, inositol 1,4,5-triphosphate; ER, endoplasmic reticulum; DAG, diacylglycerol; CaM, calmodulin; PK, protein kinase; PDE, phosphodiesterase. b) Activation with growth factor and cytokines results in the ligand-dependent receptor oligomerization and autophosphorylation at tyrosine residues. Phosphotyrosines serve as a binding site for adaptor proteins and signaling molecules resulting in the assembly of signaling platform enabling signal transduction without secondary messengers. Y-P, phosphotyrosines; ATF-2, c-MYC, c-FOS, and c-JUN, transcription factors; MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription; Jak, Janus kinase; α, β, γ, cytokine receptor subunits; IL-2, interleukin-2; PDGF, platelet-derived growth factor; SYP, synaptophysin; GAP, GTPase-activating protein; NCK, Nck adapter protein; PI3K, phosphoinositide 3-kinase; Sch, Sch protein; Grb2, growth factor receptor-bound protein 2; Src, proto-oncogene tyrosine-protein kinase Src; Raf-1 (MAPKKK), Raf-1 proto-oncogene serine/threonine-protein kinase (mitogen-activated protein kinase kinase kinase); MEK (MAPKK), mitogen-activated protein kinase kinase; Ras, RAS GTPase; SOS, son of sevenless guanine nucleotide exchange factor (adapted from [41]).
Many intracellular processes (e.g., trafficking, synthesis and degradation of fatty acids and polysaccharides) are Ca2+-driven. These processes cannot proceed via the “all-or-nothing” mechanism or based on relatively low-range fluctuations of cytosolic Ca2+, but rather depend on the broadly varying frequency of Ca2+ oscillations, which ensures gradual stepwise regulation of Ca2+-dependent intracellular events [43].
Hence, compared to neurotransmitters affecting membrane polarization, hormones act via cognate receptors by activating secondary messenger systems (cAMP, cGMP, DAG, Ca2+, etc.). Secondary messengers mediate signaling by triggering pathways for protein chemical modification (phosphorylation, ribosylation, acetylation, etc.) [44] and govern metabolic events, i.e., hormone receptors are metabotropic.
This type of interactions is characterized by receptor desensitization aimed at the cell protection against prolonged hormone action. In particular, long-lasting hormone binding (for more than 8-10 min) results in the receptor phosphorylation by the ligand-dependent protein kinase causing decrease in the receptor affinity for the ligand [45]. Extremely long (within hours) receptor stimulation initiates endocytosis resulting in the receptor downregulation (up to the receptor degradation in the lysosomes) [46]. This limits the time of hormone action down to minutes and, in some rare cases, to hours.
Lipophilic steroid hormones and thyroid hormones can cross the cell membrane into the cytosol, where they interact with the intracellular receptors and translocate to the nucleus, which prolongs their action in the cell. The hormone–receptor complex can bind to the chromatin and regulate gene expression, resulting in large-scale long-term changes in the cell phenotype [47, 48].
Our studies on the mechanisms of single-cell hormone regulation have demonstrated that hormone cascades are essential for the functioning of MSCs involved in tissue homeostasis and injury-induced regeneration [49-51]. In particular, we found that although the majority of MSCs express a broad range of hormone and neurotransmitter receptors, only a small portion of them (~5-7% of the total population) are able to respond to hormone stimulation by activating Ca2+ signaling, i.e., contain necessary intracellular signaling machinery. Moreover, a one-time exposure to the hormone results in a sharp increase in the percentage of cells able to respond to this hormone upon repeated exposure [51]. This suggested the triggering role of the subset of hormone-sensitive MSCs presumably producing biological cues that act in a paracrine manner on surrounding MSCs and augment their sensitivity to the agonist. The sensitivity of target cells can increase via increase in the membrane receptor density or upregulated expression of genes encoding intracellular signaling proteins.
We also demonstrated that a single-dose stimulation of β-adrenergic receptors in MSCs triggered a switch in the intracellular signaling from cAMP-dependent to Ca2+-driven (i.e., temporally postponed activation of the cascade initially coupled to the activated α1-adrenergic receptors, rather than caused receptor desensitization due to the excessive stimulation). This observation exemplifies the so-called heterologous sensitization, a unique mechanism for regulating MSC-specific hormone sensitivity [50, 52].
Finally, we demonstrated that the sensitivity of SCs to some hormones is associated with the heterogeneity of their differentiation potential. In particular, adipose tissue-derived MSCs were found to express all components of the renin/angiotensin/aldosterone system (RAAS). Expression of angiotensin II type 2 (AT2) receptor characterized the MSC subset displaying an augmented potential for adipocyte differentiation. These cells were also able to produce angiotensin II that presumably acted in the autocrine manner causing intracellular Ca2+ mobilization. We also observed upregulated expression of PPAR-γ and adiponectin (regulators of adipogenesis) [49] followed by adipogenic differentiation of MSCs.
The data presented above illustrate the involvement of neuroendocrine system and convincingly demonstrate its importance for rapid regulation of migration and sensitivity of a single cell at a particular moment of time. However, the duration of neurotransmitter- and hormone-related effects is remarkably shorter than the timeframe necessary for the structure recovery in the reparative regeneration lasting from several days to weeks. Another factor that limits the role of hormone regulation throughout the entire regenerative process is dynamically changing cell sensitivity (including both agonist-induced receptor desensitization and switch in the signaling cascades).
Growth factors and cytokines as unique regulators of renewal and regeneration with unique reception mechanism. As early as in 1970s, it was demonstrated that exposure to GFs and cytokines elicits more permanent (within hours to days) effect on cell migration and differentiation. This suggested that after a single exposure to these proteins, cells begin to secrete other autocrine factors that stimulate cell motility and differentiation [53, 54]. Moreover, GFs and cytokines also exhibit a prominent mitogenic effect, which is absent in the known hormones and could not be abrogated by existing inhibitors of metabotropic receptor, thus indicating existence of other signaling pathways activated by these ligands [55].
Cell sensitivity to GFs and cytokines is mediated by specialized transmembrane proteins simultaneously exhibiting receptor and enzyme activities. By now, ~60 proteins called receptor tyrosine kinases (RTKs) have been described that were classified into several families: fibroblast growth factor receptors (FGFRs), PDGF receptors (PDGFRs), VEGF receptors (VEGFRs), EGF receptors (EGFRs), and insulin and insulin-like GF receptors (IRs and IGF-1Rs) [56].
Despite certain structural differences, the majority of these receptors undergo ligand-dependent receptor dimerization followed by tyrosine autophosphorylation in the intracellular RTK domain [56, 57], which induces signal transduction (Fig. 2b).
It should be mentioned that some RTKs are able to spontaneously dimerize in the absence of GF binding. For instance, IR and IGF-1R are expressed on the cell surface as the (αβ)2 dimers linked by disulfide bonds and exhibit tyrosine kinase activity only after activation with the corresponding ligands. Similar behavior was also described for EGFR, Tie-2 (angiopoietin receptor), and some ephrin receptors that were found to assemble into large oligomers consisting of several dozens of RTKs [58]. Some authors believe that such oligomerization is necessary for the regulation of signaling after ligand binding to the receptor [59].
The response to RTK activation is very specific and determined by the position of phosphorylated tyrosine residue and multiple amplification points ensuring cooperativity in the action of several GFs and cytokines, as well as signal “interception” [56, 60]. The dependence of the phosphorylation-related effects on the position of phosphorylated tyrosine residue provides the pleotropic character of signal transduction, i.e., the opportunity to trigger different regulatory cascades from the same receptor (Fig. 2b). Phosphorylation of tyrosine residue in the receptor intracellular domain determines the binding of specific signaling molecules and subsequent activation of signaling pathways. Activated RTK can induce signaling directly or by acting as an adaptor protein necessary for the assembly of signaling complexes. Signaling via intracellular kinase cascades reaches the nucleus, where it activates transcription factors controlling gene expression, cell cycle, survival, and cell differentiation [61].
Long-term GF action is ensured by the possibility of triggering multiple signaling events upon repeated ligand-dependent assembly of the oligomeric RTK complex. RTK endocytosis can decrease the cell sensitivity to GFs and cytokines [62]; in this case, the aforementioned amplification points that converge several pathways activated via different receptor types start to play a primary role in signal transduction.
For instance, mitogen-activated protein kinase (MAP kinase) ERK mediates signaling cascades downstream of EGFR, PDGFR, and FGFR [63]. Therefore, downregulation of either of these receptors will not result in the inhibition of cell proliferation regulated by the ERK-dependent transcription factors Jun, Fos, and Myc.
Unlike hormones and neurotransmitters, GFs and cytokines are highly specific regulators of cell programming that affect cell decision to enter the cell cycle and promote cell migration or differentiation during regeneration, i.e., regulate large-scale events in the context of tissue structure. Hence, this justifies the “duplication” of signaling cascades downstream of RTKs, as well as existence of amplification checkpoints converging cascades from different GFs and cytokines. Cell differentiation, survival, proliferation, and, most importantly, formation of novel tissue structures should be protected from random action of various factors; therefore, the onset of these processes requires coordinated signaling from several receptors elicited by a combination of ligands. The reception network organized by this principle acts as a filter that dampens signaling “noise” of nonspecific or random inputs [64].
Likewise, duplication of GF and cytokine functions may play a similar role, which is a defense against temporary local conditions that can destroy molecules or alter their affinity for the receptors [65]. For example, some GFs (IGF, bFGF) may be protonated due to acidosis accompanying severe hypoxia, which results in the loss of their binding capacity [66]. However, other GFs (VEGF165, TGF-β1) are resistant to acidosis and continue to function by stimulating fibroblast proliferation and angiogenesis typical to the process of wound healing.
Another example is the loss of activity of hepatocyte growth factor (HGF) that requires proteolytic conversion of its precursor (pro-HGF) into the two-chain active isoform able to bind to the c-met receptor [67]. Kidney injury is associated with the upregulated (several-fold) expression of pro-HGF and decreased production of its proteolytic activators (HGF-A, matriptase, urokinase, etc.), which makes such response inefficient due to the reduced HGF activation. In this case, an increased production of IL-10, which also exhibits powerful anti-inflammatory and anti-fibrotic activities in protection of this vital organ, may compensate for the HGF shortage.
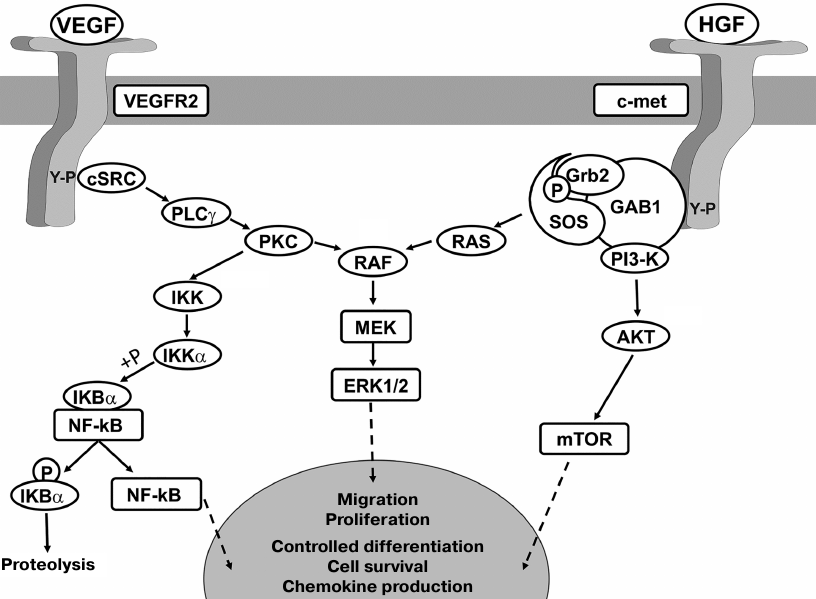
One more fact to illustrate the importance of GF pleiotropy in the regulation of regenerative processes is that during tissue recovery, VEGF and HGF act cooperatively on the proliferation and migration of endothelial cells due to the augmented ERK1/2 phosphorylation [68] but exert the opposite effects on the activation of transcription factor NF-κB (VEGF activates, whereas HGF suppresses it; see Fig. 3). NF-κB targets the downstream chemokine MCP-1 that efficiently recruits monocytes (key participants in the angio- and arteriogenesis in the heart). Therefore, despite the cooperative action of VEGF and HGF on proliferation of endothelial cells, these factors exert the opposite effects on the monocyte invasion, which promotes stabilization of de novo formed blood vessels in the tissue [69].
Fig. 3. Activation of VEGFR and c-met receptors promotes mitogenic signaling but exerts the opposite effects on cell differentiation and survival. Cell proliferation and migration are activated cell due to cooperative effect of RAF kinase phosphorylation and activation of ERK1/2 signaling. The opposite effects of VEGFR2 and c-met signaling on differentiation, production of pro-inflammatory proteins, and cell survival also depend on the activation of signaling cascades regulating transcription factors and signaling complexes affecting gene expression. Hence, RTK signaling may both promote and downregulate the same GF-induced cellular events. AKT, protein kinase B (AKT kinase); mTOR, mammalian target of rapamycin; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IKK, IκB kinase; GAB1, GRB2 associated binding protein 1.
The role of growth factors and cytokines in cell–cell communication, development, and regeneration. What are unique features of GFs and cytokines and why are the mechanisms of their reception so crucial for understanding the regulation of regeneration? The RTK system had emerged late in the evolution and has been long believed to be unique to multicellular organisms. It is believed that since the divergence between unicellular eukaryotes and ancient multicellular organisms 1.5 billion years ago, the emergence of RTK was the key step in transition to the multicellular body structure associated with a higher level of organization, i.e., cell–cell communication and organization of cells into tissues and organs [70]. An ordered system of cell-cellular interactions distinguishes organisms with obligate multicellular structure from colonies of unicellular organisms that assemble as temporary communities. In the absence of partners, each of individual organisms outside the colony relies on its own systems that sense external inputs and maintain body shape by regular duplication, which is impossible in a multicellular organism.
RTKs were found in free-living unicellular and colonial flagellate protozoa Choanoflagellatea, that represent a link between unicellular and multicellular organisms [71]. In 2001, Kind and Carroll demonstrated for the first time that Monosiga brevicollis expresses the MBRTK1 membrane protein with tyrosine kinase activity [72]. Next, it was found that the first RTKs emerged in M. brevicollis ~600 million years ago in remarkably high numbers. Monosiga brevicollis contains 128 (!) RTK genes, which reflects the primordial abundance that has emerged during adaptation, although its driving force remains unknown [73, 74].
The communication system involving GFs, cytokines, and RTKs is crucial for all stages requiring cell–cell interactions, ranging from the development to postnatal renewal and regeneration. IGF, TGF-β1, TGF-β3, HGF, PDGF-A, PDGF-B, and diverse RTKs accounting for the cell sensitivity to the above GFs, play an important role that starts already in early embryogenesis [75]. The repertoire of expressed GFs forms a network of interconnections between its components, thus determining the fate of each of them, as well as the course of organism development [56, 76]. For instance, all four blastomeres produced by zygote cleavage are profoundly different, although they have the same indistinguishable spherical shape. Each blastomere possesses unique features, as well as a set of GFs and cytokines acting in the auto- and paracrine manner in a tiny, but already non-homogenous system. Removal of cells from 4- and 8-cell mammalian embryos demonstrated that single blastomeres differ in the expression of β-catenin that plays an important role in canonical Wnt signaling pathway and regulates the differentiation state of SCs [77, 78]. Further stages of embryogenesis are characterized by an extended repertoire of generated GFs and sensitivity to them. This results in the cell lineage specialization that depends on the migration and differentiation of primitive pluripotent and then multipotent SCs [79].
From the perspective of tissue organization, regeneration is also characterized by cell division, specialization, and increased structural complexity controlled by GFs and cytokines. After injury, a temporary structure mainly containing blood clot (cells, extracellular matrix components, GFs, and cytokines) usually forms at the site of tissue damage [80]. Depending on the type, this structure may be represented by vascularized nonspecialized granulation tissue or blastema consisting of dedifferentiated somatic cells. Later, to provide a full-fledged regeneration, this agglomerate should be able to create the conditions for differentiation, proper tissue organization, and cell functioning, i.e., structural complexity [27]. Quite often this process complies with the principles similar to those acting during embryogenesis of the affected body part [81].
Progression through stages and timely switching of stimuli are necessary for tissue regeneration. All phases of reparative regeneration – from post-injury hemostasis to regeneration or fibrosis – are characterized by the activity of specialized cell types possessing unique regulatory pathways that rely on a set of specific stimulatory and inhibitory cues [41, 82].
The very early stages (thrombogenesis and inflammation) are highly conserved in vertebrates and vitally important for the host survival and fight against infections entering the site of injury. Apart from hemostasis, platelet activation is accompanied by their degranulation and release of chemokines (IL-8, IL-6, IFN-γ, etc.) that recruit neutrophils to the site of injury. This protective function is accomplished via release of toxic molecules and production of free radicals destroying microbes at the site of damage. Massive neutrophil death results in wound decontamination and creates a new gradient of cytokines (MCP-1, MIP-1, TNF-α) that attract monocytes and trigger their differentiation to macrophages. Phagocyting macrophages engulf cell debris formed by dead neutrophils and actively produce cytokines and GFs (IL-6, SDF-1α, FGF, PDGF, VEGF) that recruit to the wound site the key players of further events – MSCs, fibroblasts, and myofibroblasts [21, 41]. The same GFs stimulate cell proliferation and differentiation, as well as cell organization in an ordered structure necessary for regeneration. It is evident that already at the first stages, each cell type activated by specific cues possess unique functions and creates a repertoire of stimuli necessary for triggering and regulation of the downstream stages [83].
A good example of strictly GF-controlled regulation of developmental stages is sprouting of ectopic limbs or tail in the axolotl. Both regenerative processes require the switching between the stimuli and cooperative action of bone morphogenetic proteins (BMPs) and FGF [84]. It was found that neither FGF2 nor FGF8 or a combination of BMP2 and BMP7 were sufficient to induce limb regeneration [85]. Each of these cytokines alone induced the emergence of temporary structures on the wound surface that resembled blastema (a group of dedifferentiated cells giving rise to a new limb). Only a cooperative action of these biological cues acting in a certain order (mainly coinciding with switches in the gene expression during embryogenesis) [68] ensured the growth of the extra body part [86].
Yu et al. [87] recently demonstrated that BMP2 and BMP9 acting sequentially could induce regeneration of amputated distal phalangeal element in mammals (mice), which is undoubtedly a remarkable achievement. However, it should be noted that the basic principle of regeneration that implies sequential formation and structural complexity has not changed; regeneration will always require some cues acting in a certain sequence. The departure from this principle can explain the absence of efficacy of SC-activating molecules in clinical trials. Thus, very modest results were obtained for GFs (VEGF, bFGF, HGF, PDGF, etc.) and colony-stimulating factors (CSFs), despite the search for proper medical application of these factors – from treating burn wounds to type I diabetes mellitus [88].
Therefore, regeneration is not a function of a single cell or an effect caused by individual molecules on the processes taking place in this cell. Instead, it relies on sequential activation and regulation of diverse cell types involved in the structure recovery. This is why stimulation of regeneration should be based on the reproduction of stages of this process with consideration of the events occurring during organogenesis or tissue recovery rather than on the activity of ligands and relevant receptors involved in regeneration.
THE METHODOLOGY OF REGENERATIVE MEDICINE FROM THE VIEWPOINT OF
RENEWAL AND REGENERATION REGULATION
Gene therapy in regenerative medicine. Gene therapy (GT) is a group of methods aimed at modifying gene sequence or governing gene expression, as well as affecting the biological properties of live cells to be used for therapeutic applications. The concept of gene as a therapeutic target has been proposed long before – numerous low-molecular-weight agents and hormones can act on DNA molecules directly or indirectly, thereby affecting cell metabolism and viability. However, the idea of gene as an active principle of the therapy was proposed in early 1970s, giving rise to a new research field [89].
From the viewpoint of regeneration regulation, GT has allowed to solve the problem of the long-term expression of GFs and cytokines for the activation of formation of new structures, which normally takes a long time (days to even weeks). The long-lasting effect of these proteins could not be achieved by their local injection into tissues, as their half-life after local or systemic administration is extremely low due to proteolytic degradation and large distribution volume. Because of these factors, the concentration of GFs and cytokines rapidly declines below the receptor binding threshold, thereby preventing specific effects of these compounds on the target cells. GT has allowed to transform some organ-resident cells into producer cells manufacturing proteins that can activate SCs and trigger their proliferation and migration [90]. Plasmid and viral vectors were used to deliver GF genes (VEGF, HGF, angiopoietin 1, PDGF, etc.) to regulate tissue regeneration and its components, such as nerve and blood vessel growth, endothelial cell migration, anti-fibrotic effect, etc. [69, 91-94].
GT involving a single GF gene (VEGF, HGF, FGF, etc.) was found to be low efficient in the stimulation of regenerative processes in clinical trials [92]. The biological action of a single GF or cytokine induced the growth of some structures (blood vessels, axons), but were insufficient for the tissue formation, which required a cascade of sequentially switched signals. The clinical efficacy of this approach might be improved by using the combination GT with several GFs potentiating each other. For instance, VEGF165 and angiopoietin 1 represents one of such combinations, where VEGF165 acts as angiogenesis inducer and angiopoietin 1 acts as a chemokine recruiting pericytes and smooth muscle cells. VEGF165 triggered active capillarogenesis, but many de novo generated vessels displayed increased permeability or degraded rapidly. Angiopoietin 1 per se exhibited modest angiogenic potential but played a role of vascular stabilizer when used together with VEGF165 and reduced the side effects of VEGF monotherapy. Moreover, the VEGF165/angiopoietin 1 combination markedly augmented vascularization in the regenerating tissue compared to monotherapy with either of them [95]. However, GT has a limited potential in triggering the full-fledged tissue or organ regeneration [90, 92, 96].
It should be noted that monogene therapy was found to be efficient for the treatment of inherited disorders. The delivery of the wild-type gene variant illustrates the etiotropic GT aimed at eliminating the cause of the disease that cannot be eliminated by any other method. Because of this, scientists are getting closer to curing some inherited immunodeficiencies, enzymopathies, and hemophilia A [97-99].
Stimulation of regenerative processes by the SC secretome. Cell therapy has been long considered as an approach for developing a “stem cell-based remedy”, and over the last years, MSCs derived from various sources have been actively used for this purpose. As an easy-to-use object and a source of material for cell therapy, MSCs have been long believed to be one of the promising and safe tools for cell therapy, which has been confirmed by the data on beneficial effects of MSC application. However, in early 2000s, the studies indicating the lack of MSC integration after introduction into tissues began to emerge more often [100]. With some disappointment, some of these studies reported that MSCs were unable to integrate into the host tissue and their effects were related to the activity of their secretome [100].
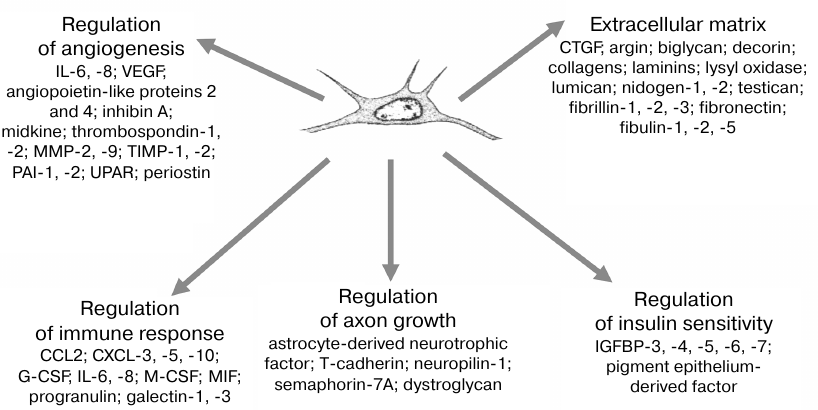
Proteomics examination of MSC secretome (including our study [101]) has demonstrated that it contained a great amount of peptide and protein components, many of which were identified as GFs, metabolic regulators, extracellular matrix components, etc. (Fig. 4) [102, 103]. Altogether, it is a complex of stimuli, which cannot be reproduced by pharmacological agents or GT methods. These data illustrated the biological role of MSCs as regulatory cells that exhibit a baseline secretory activity to ensure tissue homeostasis; however, their secretory activity can be activated upon injury in order to support regeneration. This concept is supported by the perivascular location of MSCs, which provides their simultaneous exposure to systemic (glucose, oxygen, insulin, blood hormones) and local tissue-derived cues, i.e., ensures that the cells can sense diverse stimuli produced both systemically and in situ [102, 103]. The MSC secretome was found to be a very promising tool for generating regenerative drugs that could meet the key requirements for the regeneration regulation. A combined action of GFs results in the activation of signaling at the amplification checkpoints (molecules common for different signaling pathways) by reproducing a network of cooperative cell regulation via a sum of stimuli rather than by individual molecules.
Fig. 4. Human MSC secretome and its major functional molecular groups. MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinases; PAI, plasminogen activator inhibitor; UPAR, urokinase plasminogen activator surface receptor; CCL2, C-C motif chemokine ligand 2; CXCL, chemokine (C-X-C motif) ligand; G-CSF, granulocyte-colony stimulating factor; M-CSF, macrophage colony-stimulating factor; IGFBP, insulin-like growth factor-binding protein.
This has provided the basis for developing regenerative drugs containing MSC-produced proteins intended for the stimulation of tissue recovery. Our in vivo studies showed that the MSC secretome displayed a potent antiangiogenic and neurotrophic activity by stimulating the healing of burn wounds and restoring spermatogenesis [101, 104].
One of the drugs is Thero-101 (previously called NeuroFX), an infusion preparation containing purified human MSC-secreted proteins intended for the treatment of consequences of post-ischemic stroke. Currently, the pharmaceutical company manufacturing this preparation is starting the first clinical trial for assessing its efficacy.
It should be also mentioned that numerous pharmacological effects of the MSC secretome are associated not only with proteins and peptides, but also with extracellular vesicles, such as microvesicles and exosomes [105]. For a long time, extracellular vesicles had been considered as the products of removal of obsolete organelles and misfolded proteins from the cells. However, it can be now stated with certainty that extracellular vesicles serve as means of cell–cell communication. Moreover, because these vesicles contain nucleic acids (mRNAs, microRNAs, etc.), they may be also involved in the horizontal transfer of genetic information in human body, including during stimulated cell differentiation and transdifferentiation [106].
Therefore, from the viewpoint of regeneration, introduced MSCs are indeed unable to integrate into the host tissues; however, they can influence most of the of post-injury recovery phases via their secretory activity [104].
MSCs introduced into the injured tissues can sense the microenvironment. For instance, MSCs can specifically modify their secretome in response to hypoxia or high concentrations of pro-inflammatory cytokines (e.g., IL-8 and TNF-α), thus displaying the adaptive properties [107, 108]. They can also accelerate the relief of inflammation in the acute and peracute phases and promote the growth of capillaries and nerve endings necessary for the regeneration and tissue recovery at the later stage. It should be noted that under the influence of tissue environment, MSCs may adopt the myofibroblast phenotype to enhance fibrosis and scarring at the site of injury [109, 110].
Hence, the secretory activity of MSCs may be used for creating the secretome-based preparations. However, such preparations would represent a cocktail of released factors and, therefore, cannot be used for reproducing the sequential process of regeneration, the importance of which has been discussed above. MSCs introduced into the tissues are able to adaptively change their secretory activity depending on the environment. However, some data suggest MSC participation in fibrogenesis in injured organs. Both these pathways have their own prospects and will probably find application in the future. At present, the state of MSCs and other types of cells can be regulated in vitro. The secretome composition may be efficiently changed by mimicking shear stress, exposure to activating molecules, or hypoxia, which can be used for obtaining cell-free preparations of various modalities for the treatment of a wide range of human disorders.
Tissue-specific SCs and their niche as therapeutic targets. Tissue-specific SCs have been long believed to be very promising agents for regenerative medicine. They are found in all human parenchymal organs, lungs, skin, intestinal crypts, etc. These postnatal SCs exist as a limited population of undifferentiated cells retained during embryogenesis and involved in tissue renewal and regeneration [111].
However, it has been repeatedly found that tissue-derived SCs are unable to give rise to tissues or to form their fully functional equivalents. In some cases, the problem was partially solved by tissue engineering or use of molecular scaffolds (synthetic matrix or decellularized organs) [112]. Moreover, some tissue-specific SCs cannot be induced ex vivo even at the early stages of organogenesis. For instance, skeletal muscle satellite cells can be activated in vitro to express transcription factors responsible for mitogenesis and assembly of myofibril-like structures [113]. However, it is impossible to induce their regulated contractile activity for further integration into the tissues [114].
These are two possible explanations to these facts: (i) by losing the contact with their environment, SCs also lose the ability for differentiation; (ii) after reaching the committed precursor stage, SCs undergoing differentiation require full-featured environment to mature into terminally differentiated state and to integrate into the tissue both morphologically and functionally [21, 22].
Hence, we can conclude that the functional unit in regeneration is a combination of SCs and their specific regulatory environment rather than SCs per se and their intrinsic properties. According to the modern concept, this environment, named the niche, senses the activating cues and determines the fate of SCs at the early stages of differentiation [115]. The anatomy of various types of niches and their regulation have been discussed in numerous comprehensive reviews; here, we would only like to mention that along with SCs, the niche also contains soluble factors, extracellular matrix components, and supporting cells. Being the supporting cells, MSCs are involved in both maintaining SCs in the quiescent state and their activation. MSCs also contribute to the completion of regeneration at stage of formation of tissue-specific extracellular matrix, as well as vascular and neural components [116].
Coming back to the topic of this review, we believe that elucidation of the mechanisms behind the SC niche functioning and development of approaches for its controlled activation or post-injury recovery will become a priority in the future regenerative medicine. It is possible that such approaches will be based on sequential switching from the factors and stimuli activating SCs to those maintaining SC maturation during regeneration (i.e., on regulatory principles described in the first section of this review).
Therefore, it will be possible to omit the ex vivo culturing of SCs, which poses a risk of microbial contamination, chromosomal aberrations, and genetic rearrangements resulting in the loss of SC regenerative properties shaped by niche. Undoubtfully, in some cases, SCs and surrounding cells initially possess low regenerative potential; the SC pool becomes exhausted upon aging, and the physiological functions of SC are adversely affected by metabolic and cardiovascular diseases [117]. In this case, we have to achieve deeper understanding of the processes occurring in the SC niche, as well as the how the adverse factors might affect it. As a result, this will help in creating a foundation for regulating the SC niche and allow to develop new approaches for blocking or suppressing the formation of pathological niches, e.g., cancer SC niches [118].
“Small molecules” in regenerative medicine. Here, we have approached an important direction in the regenerative medicine – regulated tissue renewal and regeneration in situ. In this context, we have to mention “small molecules”, e.g., specific inhibitors targeting proteins and regulatory pathways controlling the state of SCs. The FX-322 drug representing a combination of two low-molecular-weight inhibitors is now entering the clinical trial stage in the USA. In this drug, inhibitor 1 targets glycogen synthase kinase 3 (GSK3); inhibition of GSK3 results in the activation of Wnt signaling (key regulatory pathway for many progenitor cells). Inhibitor 2 blocks the activity of histone deacetylase 1 (HDAC1), thereby decreasing its twisting effects on the DNA molecules. The cumulative effect of these two inhibitors is activation of Lgr5+ progenitor cells (auditory hair cell precursors). These cells are now often found to die in the early age due to broad use of earphones and personal audio devices. In particular, FX-322 applied intratympanically in the middle ear activates Lgr5+ progenitor cells resulting in the recovery of auditory sensory epithelium and hearing. This inhibitor cocktail may be also efficient in the intestinal crypt niche, where epithelial regeneration also depends on the activity of Lgr5+ cells. Finally, Lgr5+ cells were found in the hair follicle cells, suggesting that FX-322 can be used for the treatment of different types of alopecia.
This example illustrates that a well-chosen combination of pharmacological inhibitors may be an efficient inducer of regeneration via acting on tissue-specific SCs and can allow to avoid the culturing of these cells ex vivo. Coming back to the first section of the review, it should be noted that in this case, the mechanism of drug action was inhibition of molecular targets rather than their activation, as it happens for the MSC secretome or proteins produced after the gene delivery to the tissue.
However, the strategy described above has certain limitations, such as exhaustion of the pool of tissue-specific SCs, which may be prevented by activation of cell proliferation via virus-mediated delivery of genes encoding positive cell cycle regulators or regulatory RNAs causing limited dedifferentiation of mature cells followed by their proliferation and subsequent redifferentiation. Moreover, this approach may be combined with the above strategy, as it was done in the experiments on the activation of proliferation of mature mouse cardiomyocytes [119]. It was shown that intracardiac introduction of genes for four cell cycle regulators [cyclin-dependent kinase 1 (CDK1), CDK4, cyclin B1 (CCNB1), and cyclin D1 (CCND1)] triggered cardiomyocyte proliferation. Moreover, the use of two low-molecular-weight inhibitors suppressing the activity of TGF-β1 and kinase Wee-1 allowed to achieve similar effect after introducing CDK4 and CCND1 genes only.
Another promising approach is cell transdifferentiation in situ via introduction of transcription factors or regulatory RNAs delivered by viral vectors or exosomes. For example, fibroblasts were directly reprogrammed into hepatocytes, cardiomyocytes, and dermal cells avoiding the pluripotent stage [34, 35, 120].
Currently, the scientists have closely approached the development of methods based on the combination of GT and pharmacological regulation of differentiation, which might be used to activate tissue renewal and regeneration after injury.
The studies of regenerative processes, no matter how difficult the subject of these studies seems to be, have already provided the groundbreaking results. In the XXI century, in less than 20 years, it has become possible to achieve regeneration of heart and limb parts in mammals, to unveil the patterns behind the loss of postnatal capacity for reparative regeneration, and finally, to discover ~20 new targets responsible for the postnatal functioning of SCs. Extending the methodological base – from relevant animal models to single-cell RNA sequencing – has provided unique opportunities in regenerative biology and medicine.
In this review, we presented the regulatory systems currently examined in regenerative medicine. This direction of studies has come to the point when the most rational approach will convergence of regenerative medicine with regenerative biology (from which it has borrowed many terms and some methodology). Exploring molecular mechanisms behind SC regulation has been a leading direction in this field and provided numerous promising technologies. However, the current task is understanding of how post-injury cells in the postnatal period can reproduce the processes of tissue organization occurring in embryogenesis.
The fact that human body lacks evolutionarily developed blockade of regeneration, as all cell mechanisms typical to animals capable of efficient recovery of body parts may also occur in the post-injury tissues in Homo sapiens, inspires some optimism in researchers. Apart from proliferation of mature cells and SC differentiation, dedifferentiation events also take place, including direct conversion of one cell type into another after injury. Hence, an important task of regenerative biology and medicine for the next decade will be examination of regenerating tissues as a maturing network of GF- and cytokine-mediated cell–cell interactions (analogous to that existing in embryogenesis). Although implementation of correct program for this process has long been associated with activated resident SCs, currently growing attention has been attracted to the niche providing specific regulatory environment of SCs. The results of such studies have already found their practical application, as the methods for triggering of ex vivo cell culture-free regeneration (i.e., in situ) by using small molecules, GT, and postnatal SC secretome have been developed.
Undoubtedly, investigation of metabolic regulation in regeneration and regulatory cascades providing signal transduction and cell fate determination remains important. Although we expect that in the nearest future, the new targets will be identified using classical approaches of cell biology and biochemistry, expanding application of gene engineering and bioinformatics methods will markedly improve the efficiency of search for these targets and their rapid testing in relevant model objects.
Funding. The study was supported by the Russian Science Foundation (project 19-75-30007; manuscript preparation and providing access to source materials) and Grant of the President of the Russian Federation for Governmental Support of Young Russian Scientists (MK-1068.2019.7; figure preparation).
Conflict of interest. The authors declare no conflict of interest.
Compliance with ethical standards. The article does not contain description of studies with human subjects or animals performed by any of the authors.
REFERENCES
1.Goss, R. J. (1969) Principles of
Regeneration, Academic Press, New York.
2.Wyllie, A. H. (1987) Apoptosis: cell death in
tissue regulation, J. Pathol., 153, 313-316.
3.Guillot, C., and Lecuit, T. (2013) Mechanics of
epithelial tissue homeostasis and morphogenesis, Science,
340, 1185-1189.
4.Alberts, B., Johnson, A., Lewis, J., Raff, M.,
Roberts, K., and Walter, P. (2002) Molecular Biology of the
Cell, 4th Edn., Garland Science, New York.
5.Iismaa, S. E., Kaidonis, X., Nicks, A. M., Bogush,
N., Kikuchi, K., Naqvi, N., Harvey, R. P., Husain, A., and Graham, R.
M. (2018) Comparative regenerative mechanisms across different
mammalian tissues, NPJ Regen. Med., 3, 6.
6.Stocum, D. L. (2012) An overview of regenerative
biology, in Regenerative Biology and Medicine, 2nd Edn.,
Academic Press, San Diego, pp. 3-20.
7.Goss, R. J. (1980) Prospects of regeneration in
man, Clin. Orthop. Relat. Res., 151, 270-282.
8.Seifert, A. W., and Muneoka, K. (2018) The blastema
and epimorphic regeneration in mammals, Dev. Biol., 433,
190-199.
9.Bleacher, J. C., Adolph, V. R., Dillon, P. W., and
Krummel, T. M. (1993) Fetal tissue repair and wound healing,
Dermatol. Clin., 11, 677-683.
10.Dostal, G. H., and Gamelli, R. L. (1993) Fetal
wound healing, Surg. Gynecol. Obstet., 176, 299-306.
11.Burrington, J. D. (1971) Wound healing in the
fetal lamb, J. Pediatr. Surg., 6, 523-528.
12.Frantz, F. W., Diegelmann, R. F., Mast, B. A.,
and Cohen, I. K. (1992) Biology of fetal wound healing: collagen
biosynthesis during dermal repair, J. Pediatr. Surg., 27,
945-948.
13.Buonocore, G., Perrone, S., and Tataranno, M. L.
(2017) Oxidative stress in the newborn, Oxid. Med. Cell.
Longev., 2017, 1094247.
14.Torres-Cuevas, I., Parra-Llorca, A.,
Sanchez-Illana, A., Nunez-Ramiro, A., Kuligowski, J., Chafer-Pericas,
C., Cernada, M., Escobar, J., and Vento, M. (2017) Oxygen and oxidative
stress in the perinatal period, Redox. Biol., 12,
674-681.
15.Yun, M. H. (2015) Changes in regenerative
capacity through lifespan, Int. J. Mol. Sci., 16,
25392-25432.
16.Nakada, Y., Canseco, D. C., Thet, S., Abdisalaam,
S., Asaithamby, A., Santos, C. X., Shah, A. M., Zhang, H., Faber, J.
E., Kinter, M. T., Szweda, L. I., Xing, C., Hu, Z., Deberardinis, R.
J., Schiattarella, G., Hill, J. A., Oz, O., Lu, Z., Zhang, C. C.,
Kimura, W., and Sadek, H. A. (2017) Hypoxia induces heart regeneration
in adult mice, Nature, 541, 222-227.
17.Simkin, J., and Seifert, A. W. (2018) Concise
review: translating regenerative biology into clinically relevant
therapies: are we on the right path? Stem Cells Transl. Med.,
7, 220-231.
18.Gawriluk, T. R., Simkin, J., Thompson, K. L.,
Biswas, S. K., Clare-Salzler, Z., Kimani, J. M., Kiama, S. G., Smith,
J. J., Ezenwa, V. O., and Seifert, A. W. (2016) Comparative analysis of
ear-hole closure identifies epimorphic regeneration as a discrete trait
in mammals, Nat. Commun., 7, 11164.
19.Christ, G. J., Saul, J. M., Furth, M. E., and
Andersson, K. E. (2013) The pharmacology of regenerative medicine,
Pharmacol. Rev., 65, 1091-1133.
20.Tanaka, E. M., and Reddien, P. W. (2011) The
cellular basis for animal regeneration, Dev. Cell, 21,
172-185.
21.Nimiritsky, P. P., Eremichev, R. Y.,
Alexandrushkina, N. A., Efimenko, A. Y., Tkachuk, V. A., and
Makarevich, P. I. (2019) Unveiling mesenchymal stromal cells'
organizing function in regeneration, Int. J. Mol. Sci.,
20, E823, doi: 10.3390/ijms20040823.
22.Nimiritsky, P. P., Sagaradze, G. D., Efimenko, A.
Y., Makarevich, P. I., and Tkachuk, V. A. (2018) The stem cell niche,
Tsitologiya, 60, 575-586.
23.Mazzarello, P. (1999) A unifying concept: the
history of cell theory, Nat. Cell Biol., 1, E13-E15.
24.Shay, J. W., and Wright, W. E. (2000) Hayflick,
his limit, and cellular ageing, Nat. Rev. Mol. Cell Biol.,
1, 72-76.
25.Munoz-Espin, D., and Serrano, M. (2014) Cellular
senescence: from physiology to pathology, Nat. Rev. Mol. Cell
Biol., 15, 482-496.
26.Klochendler, A., Weinberg-Corem, N., Moran, M.,
Swisa, A., Pochet, N., Savova, V., Vikesa, J., Van de Peer, Y.,
Brandeis, M., Regev, A., Nielsen, F. C., Dor, Y., and Eden, A. (2012) A
transgenic mouse marking live replicating cells reveals in vivo
transcriptional program of proliferation, Dev. Cell, 23,
681-690.
27.Brockes, J. P., and Kumar, A. (2008) Comparative
aspects of animal regeneration, Annu. Rev. Cell Dev. Biol.,
24, 525-549.
28.Wang, W. E., Li, L., Xia, X., Fu, W., Liao, Q.,
Lan, C., Yang, D., Chen, H., Yue, R., Zeng, C., Zhou, L., Zhou, B.,
Duan, D. D., Chen, X., Houser, S. R., and Zeng, C. (2017)
Dedifferentiation, proliferation, and redifferentiation of adult
mammalian cardiomyocytes after ischemic injury, Circulation,
136, 834-848.
29.Jopling, C., Sleep, E., Raya, M., Marti, M.,
Raya, A., and Izpisua Belmonte, J. C. (2010) Zebrafish heart
regeneration occurs by cardiomyocyte dedifferentiation and
proliferation, Nature, 464, 606-609.
30.Takahashi, K. (2014) Cellular reprogramming,
Cold Spring Harb. Perspect. Biol., 6, a018606, doi:
10.1101/cshperspect.a018606.
31.Kelaini, S., Cochrane, A., and Margariti, A.
(2014) Direct reprogramming of adult cells: avoiding the pluripotent
state, Stem Cells Cloning, 7, 19-29.
32.Criscimanna, A., Speicher, J. A., Houshmand, G.,
Shiota, C., Prasadan, K., Ji, B., Logsdon, C. D., Gittes, G. K., and
Esni, F. (2011) Duct cells contribute to regeneration of endocrine and
acinar cells following pancreatic damage in adult mice,
Gastroenterology, 141, 1451-1462, doi:
10.1053/j.gastro.2011.07.003.
33.Beer, R. L., Parsons, M. J., and Rovira, M.
(2016) Centroacinar cells: at the center of pancreas regeneration,
Dev. Biol., 413, 8-15.
34.Raven, A., Lu, W. Y., Man, T. Y.,
Ferreira-Gonzalez, S., O'Duibhir, E., Dwyer, B. J., Thomson, J. P.,
Meehan, R. R., Bogorad, R., Koteliansky, V., Kotelevtsev, Y.,
Ffrench-Constant, C., Boulter, L., and Forbes, S. J. (2017)
Cholangiocytes act as facultative liver stem cells during impaired
hepatocyte regeneration, Nature, 547, 350-354.
35.Malato, Y., Naqvi, S., Schurmann, N., Ng, R.,
Wang, B., Zape, J., Kay, M. A., Grimm, D., and Willenbring, H. (2011)
Fate tracing of mature hepatocytes in mouse liver homeostasis and
regeneration, J. Clin. Invest., 121, 4850-4860.
36.Discher, D. E., Mooney, D. J., and Zandstra, P.
W. (2009) Growth factors, matrices, and forces combine and control stem
cells, Science, 324, 1673-1677.
37.Michalopoulos, G. K. (1990) Liver regeneration:
molecular mechanisms of growth control, FASEB J., 4,
176-187.
38.Michalopoulos, G. K., and DeFrances, M. C. (1997)
Liver regeneration, Science, 276, 60-66.
39.Tkachuk, V. A. (1994) Physiology of endocrine
system, Usp. Fiziol. Nauk, 25, 47-54.
40.Tkachuk, V. A. (1987) A role and place for cyclic
nucleotides in neuroendocrine regulation in cells and tissues,
Nauchnye Doki. Vyss. Shkoly Biol. Nauki, 6, 5-17.
41.Tkachuk, V. A., Vorotnikov, A. V., and
Tyurin-Kuz’min, P. A. (2017) Basics of Molecular
Endocrinology: Sensing and Intracellular Signaling [in Russian],
GEOTAR-Media, Moscow.
42.Grigorian, G. Y., Mirzapoyazova, T. Y., Resink,
T. J., Danilov, S. M., and Tkachuk, V. A. (1989) Regulation of
phosphoinositide turnover in endothelium from human pulmonary artery,
aorta and umbilical vein. Antagonistic action on the beta-adrenoceptor
coupled adenylate cyclase system, J. Mol. Cell. Cardiol., 21
(Suppl. 1), 119-123.
43.Tkachuk, V. A. (2000) Membrane receptors and
intracellular calcium, Membr. Cell Biol., 13,
263-285.
44.Tkachuk, V. A., and Avakyan, A. E. (2003)
Molecular mechanisms for coupling G-proteins to membrane receptors and
secondary messenger systems, Ross. Fiziol. Zh. I. M. Sechenova,
89, 1478-1490.
45.Tkachuk, V. A. (1982) Regulation of adenylate
cyclase by hormones and guanine nucleotides in normal, desensitized,
and resensitized rabbit heart, Adv. Myocardiol., 3,
305-316.
46.Tkachuk, V. A. (1989) Developing
hormone-triggered desensitization and hypersensitivity in cardiac
adenylate cyclase, Kardiologiya, 29, 122-125.
47.Tkachuk, V. A., Rybin, V. O., and Nikashin, A. V.
(1994) Steroid and thyroid hormones in regulating G-proteins linking
membrane receptors to the secondary messenger systems, Dokl. Ross.
Akad. Med. Nauk, No. 12, 7-12.
48.Evans, R. M., and Mangelsdorf, D. J. (2014)
Nuclear receptors, RXR, and the big bang, Cell, 157,
255-266.
49.Sysoeva, V. Y., Ageeva, L. V., Tyurin-Kuzmin, P.
A., Sharonov, G. V., Dyikanov, D. T., Kalinina, N. I., and Tkachuk, V.
A. (2017) Local angiotensin II promotes adipogenic differentiation of
human adipose tissue mesenchymal stem cells through type 2 angiotensin
receptor, Stem Cell Res., 25, 115-122.
50.Tyurin-Kuzmin, P. A., Chechekhin, V. I., Ivanova,
A. M., Dyikanov, D. T., Sysoeva, V. Y., Kalinina, N. I., and Tkachuk,
V. A. (2018) Noradrenaline sensitivity is severely impaired in
immortalized adipose-derived mesenchymal stem cell line, Int. J.
Mol. Sci., 19, E3712, doi: 10.3390/ijms19123712.
51.Tyurin-Kuzmin, P. A., Fadeeva, J. I.,
Kanareikina, M. A., Kalinina, N. I., Sysoeva, V. Y., Dyikanov, D. T.,
Stambolsky, D. V., and Tkachuk, V. A. (2016) Activation of
beta-adrenergic receptors is required for elevated
alpha1A-adrenoreceptors expression and signaling in mesenchymal stromal
cells, Sci. Rep., 6, 32835.
52.Tyurin-Kuzmin, P. A., Dyikanov, D. T., Fadeeva,
J. I., Sysoeva, V. Y., and Kalinina, N. I. (2018) Flow cytometry
analysis of adrenoceptors expression in human adipose-derived
mesenchymal stem/stromal cells, Sci. Data, 5, 180196.
53.Levi-Montalcini, R., and Booker, B. (1960)
Excessive growth of the sympathetic ganglia evoked by a protein
isolated from mouse salivary glands, Proc. Natl. Acad. Sci. USA,
46, 373-384.
54.Cohen, S. (1962) Isolation of a mouse
submaxillary gland protein accelerating incisor eruption and eyelid
opening in the new-born animal, J. Biol. Chem., 237,
1555-1562.
55.Cohen, S. (2008) Origins of growth factors: NGF
and EGF, J. Biol. Chem., 283, 33793-33797.
56.Lemmon, M. A., and Schlessinger, J. (2010) Cell
signaling by receptor tyrosine kinases, Cell, 141,
1117-1134.
57.Hunter, T. (2015) Discovering the first tyrosine
kinase, Proc. Natl. Acad. Sci. USA, 112, 7877-7882.
58.Clayton, A. H., Walker, F., Orchard, S. G.,
Henderson, C., Fuchs, D., Rothacker, J., Nice, E. C., and Burgess, A.
W. (2005) Ligand-induced dimer–tetramer transition during the
activation of the cell surface epidermal growth factor receptor –
a multidimensional microscopy analysis, J. Biol. Chem.,
280, 30392-30399.
59.Himanen, J. P., and Nikolov, D. B. (2003) Eph
signaling: a structural view, Trends Neurosci., 26,
46-51.
60.Fambrough, D., McClure, K., Kazlauskas, A., and
Lander, E. S. (1999) Diverse signaling pathways activated by growth
factor receptors induce broadly overlapping, rather than independent,
sets of genes, Cell, 97, 727-741.
61.Katz, M., Amit, I., and Yarden, Y. (2007)
Regulation of MAPKs by growth factors and receptor tyrosine kinases,
Biochim. Biophys. Acta, 1773, 1161-1176.
62.Goh, L. K., and Sorkin, A. (2013) Endocytosis of
receptor tyrosine kinases, Cold Spring Harb. Perspect. Biol.,
5, a017459.
63.Roskoski, R., Jr. (2012) ERK1/2 MAP kinases:
structure, function, and regulation, Pharmacol. Res., 66,
105-143.
64.Ihle, J. N. (1995) Cytokine receptor signalling,
Nature, 377, 591-594.
65.D'Arcangelo, D., Facchiano, F., Barlucchi, L. M.,
Melillo, G., Illi, B., Testolin, L., Gaetano, C., and Capogrossi, M. C.
(2000) Acidosis inhibits endothelial cell apoptosis and function and
induces basic fibroblast growth factor and vascular endothelial growth
factor expression, Circ. Res., 86, 312-318.
66.Green, J., and Maor, G. (2000) Effect of
metabolic acidosis on the growth hormone/IGF-I endocrine axis in
skeletal growth centers, Kidney Int., 57, 2258-2267.
67.Conway, K., Price, P., Harding, K. G., and Jiang,
W. G. (2006) The molecular and clinical impact of hepatocyte growth
factor, its receptor, activators, and inhibitors in wound healing,
Wound Repair Regen., 14, 2-10.
68.Satoh, A., and Makanae, A. (2014) Conservation of
position-specific gene expression in axolotl limb skin, Zool.
Sci., 31, 6-13.
69.Makarevich, P. I., Dergilev, K. V., Tsokolaeva,
Z. I., Boldyreva, M. A., Shevchenko, E. K., Gluhanyuk, E. V.,
Gallinger, J. O., Menshikov, M. Y., and Parfyonova, Y. V. (2018)
Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene
therapy in a rat model of myocardial infarction, PLoS One,
13, e0197566.
70.Rokas, A. (2008) The molecular origins of
multicellular transitions, Curr. Opin. Genet. Dev., 18,
472-478.
71.King, N. (2004) The unicellular ancestry of
animal development, Dev. Cell, 7, 313-325.
72.King, N., and Carroll, S. B. (2001) A receptor
tyrosine kinase from choanoflagellates: molecular insights into early
animal evolution, Proc. Natl. Acad. Sci. USA, 98,
15032-15037.
73.Pincus, D., Letunic, I., Bork, P., and Lim, W. A.
(2008) Evolution of the phosphotyrosine signaling machinery in
premetazoan lineages, Proc. Natl. Acad. Sci. USA, 105,
9680-9684.
74.Manning, G., Young, S. L., Miller, W. T., and
Zhai, Y. (2008) The protist, Monosiga brevicollis, has a
tyrosine kinase signaling network more elaborate and diverse than found
in any known metazoan, Proc. Natl. Acad. Sci. USA, 105,
9674-9679.
75.Mummery, C. L., van den Eijnden-van Raaij, A. J.,
Feijen, A., Freund, E., Hulskotte, E., Schoorlemmer, J., and Kruijer,
W. (1990) Expression of growth factors during the differentiation of
embryonic stem cells in monolayer, Dev. Biol., 142,
406-413.
76.Shilo, B. Z. (2005) Regulating the dynamics of
EGF receptor signaling in space and time, Development,
132, 4017-4027.
77.Piotrowska-Nitsche, K., Perea-Gomez, A.,
Haraguchi, S., and Zernicka-Goetz, M. (2005) Four-cell stage mouse
blastomeres have different developmental properties,
Development, 132, 479-490.
78.Zdravkovic, T., Nazor, K. L., Larocque, N.,
Gormley, M., Donne, M., Hunkapillar, N., Giritharan, G., Bernstein, H.
S., Wei, G., Hebrok, M., Zeng, X., Genbacev, O., Mattis, A., McMaster,
M. T., Krtolica, A., Valbuena, D., Simon, C., Laurent, L. C., Loring,
J. F., and Fisher, S. J. (2015) Human stem cells from single
blastomeres reveal pathways of embryonic or trophoblast fate
specification, Development, 142, 4010-4025.
79.Manca, A., Capsoni, S., Di Luzio, A., Vignone,
D., Malerba, F., Paoletti, F., Brandi, R., Arisi, I., Cattaneo, A., and
Levi-Montalcini, R. (2012) Nerve growth factor regulates axial rotation
during early stages of chick embryo development, Proc. Natl. Acad.
Sci. USA, 109, 2009-2014.
80.Ud-Din, S., Volk, S. W., and Bayat, A. (2014)
Regenerative healing, scar-free healing and scar formation across the
species: current concepts and future perspectives, Exp.
Dermatol., 23, 615-619.
81.Bielefeld, K. A., Amini-Nik, S., and Alman, B. A.
(2013) Cutaneous wound healing: recruiting developmental pathways for
regeneration, Cell. Mol. Life Sci., 70, 2059-2081.
82.Kalinina, N. I., Sysoeva, V. Y., Rubina, K. A.,
Parfenova, Y. V., and Tkachuk, V. A. (2011) Mesenchymal stem cells in
tissue growth and repair, Acta Naturae, 3, 30-37.
83.Takeo, M., Lee, W., and Ito, M. (2015) Wound
healing and skin regeneration, Cold Spring Harb. Perspect. Med.,
5, a023267.
84.Makanae, A., Mitogawa, K., and Satoh, A. (2016)
Cooperative inputs of Bmp and Fgf signaling induce tail regeneration in
urodele amphibians, Dev. Biol., 410, 45-55.
85.Makanae, A., Hirata, A., Honjo, Y., Mitogawa, K.,
and Satoh, A. (2013) Nerve independent limb induction in axolotls,
Dev. Biol., 381, 213-226.
86.Makanae, A., Mitogawa, K., and Satoh, A. (2014)
Implication of two different regeneration systems in limb regeneration,
Regeneration (Oxf.), 1, 1-9.
87.Yu, L., Dawson, L. A., Yan, M., Zimmel, K., Lin,
Y. L., Dolan, C. P., Han, M., and Muneoka, K. (2019) BMP9 stimulates
joint regeneration at digit amputation wounds in mice, Nat.
Commun., 10, 424.
88.Barrientos, S., Brem, H., Stojadinovic, O., and
Tomic-Canic, M. (2014) Clinical application of growth factors and
cytokines in wound healing, Wound Repair Regen., 22,
569-578.
89.Friedmann, T., and Roblin, R. (1972) Gene therapy
for human genetic disease? Science, 175, 949-955.
90.Makarevich, P. I., and Parfyonova, Ye. V. (2017)
Therapeutic angiogenesis: foundations and practical application, in
Physiologic and Pathologic Angiogenesis – Signaling Mechanisms
and Targeted Therapy, Intech Open, London, pp. 343-364.
91.Boldyreva, M. A., Bondar, I. V., Stafeev, I. S.,
Makarevich, P. I., Beloglazova, I. B., Zubkova, E. S., Shevchenko, E.
K., Molokotina, Y. D., Karagyaur, M. N., Ratner, E. I., and Parfyonova,
Y. V. (2018) Plasmid-based gene therapy with hepatocyte growth factor
stimulates peripheral nerve regeneration after traumatic injury,
Biomed. Pharmacother., 101, 682-690.
92.Makarevich, P. I., Rubkina, K. A., Dyykanov, D.
T., Tkachuk, V. A., and Parfenova, E. V. (2015) A therapeutic
angiogenesis induced by growth factors: current state and perspectives,
Kardiologiya, 55, 59-71.
93.Karagyaur, M., Dyikanov, D., Makarevich, P.,
Semina, E., Stambolsky, D., Plekhanova, O., Kalinina, N., and Tkachuk,
V. (2015) Non-viral transfer of BDNF and uPA stimulates peripheral
nerve regeneration, Biomed. Pharmacother., 74, 63-70.
94.Makarevich, P., Tsokolaeva, Z., Shevelev, A.,
Rybalkin, I., Shevchenko, E., Beloglazova, I., Vlasik, T., Tkachuk, V.,
and Parfyonova, Y. (2012) Combined transfer of human VEGF165 and HGF
genes renders potent angiogenic effect in ischemic skeletal muscle,
PLoS One, 7, e38776.
95.Shyu, K. G., Chang, H., and Isner, J. M. (2003)
Synergistic effect of angiopoietin-1 and vascular endothelial growth
factor on neoangiogenesis in hypercholesterolemic rabbit model with
acute hindlimb ischemia, Life Sci., 73, 563-579.
96.Slobodkina, E. A., Makarevich, P. I., and
Dolinkin, A. O. (2018) Development of gene-therapy preparations,
Biofarmatsevt. Zh., 10, 3-14.
97.Badat, M., and Davies, J. (2017) Gene therapy in
a patient with sickle cell disease, N. Engl. J. Med.,
376, 2093-2094.
98.Rangarajan, S., Walsh, L., Lester, W., Perry, D.,
Madan, B., Laffan, M., Yu, H., Vettermann, C., Pierce, G. F., Wong, W.
Y., and Pasi, K. J. (2017) AAV5-factor VIII gene transfer in severe
hemophilia A, N. Engl. J. Med., 377, 2519-2530.
99.Dunbar, C. E., High, K. A., Joung, J. K., Kohn,
D. B., Ozawa, K., and Sadelain, M. (2018) Gene therapy comes of age,
Science, 359, eaan4672, doi: 10.1126/science.aan4672.
100.Savukinas, U. B., Enes, S. R., Sjoland, A. A.,
and Westergren-Thorsson, G. (2016) Concise review: the bystander
effect: mesenchymal stem cell-mediated lung repair, Stem Cells,
34, 1437-1444.
101.Kalinina, N., Kharlampieva, D., Loguinova, M.,
Butenko, I., Pobeguts, O., Efimenko, A., Ageeva, L., Sharonov, G.,
Ischenko, D., Alekseev, D., Grigorieva, O., Sysoeva, V., Rubina, K.,
Lazarev, V., and Govorun, V. (2015) Characterization of secretomes
provides evidence for adipose-derived mesenchymal stromal cells
subtypes, Stem Cell Res. Ther., 6, 221.
102.Rolandsson Enes, S., Ahrman, E., Palani, A.,
Hallgren, O., Bjermer, L., Malmstrom, A., Scheding, S., Malmstrom, J.,
and Westergren-Thorsson, G. (2017) Quantitative proteomic
characterization of lung-MSC and bone marrow-MSC using DIA-mass
spectrometry, Sci. Rep., 7, 9316.
103.Kim, H. S., Choi, D. Y., Yun, S. J., Choi, S.
M., Kang, J. W., Jung, J. W., Hwang, D., Kim, K. P., and Kim, D. W.
(2012) Proteomic analysis of microvesicles derived from human
mesenchymal stem cells, J. Proteome Res., 11,
839-849.
104.Sagaradze, G., Grigorieva, O., Nimiritsky, P.,
Basalova, N., Kalinina, N., Akopyan, Z., and Efimenko, A. (2019)
Conditioned medium from human mesenchymal stromal cells: towards the
clinical translation, Int. J. Mol. Sci., 20, E1656, doi:
10.3390/ijms20071656.
105.Phinney, D. G., and Pittenger, M. F. (2017)
Concise review: MSC-derived exosomes for cell-free therapy, Stem
Cells, 35, 851-858.
106.Bang, O. Y., and Kim, E. H. (2019) Mesenchymal
stem cell-derived extracellular vesicle therapy for stroke: challenges
and progress, Front. Neurol., 10, 211.
107.Zubkova, E. S., Beloglazova, I. B., Makarevich,
P. I., Boldyreva, M. A., Sukhareva, O. Y., Shestakova, M. V., Dergilev,
K. V., Parfyonova, Y. V., and Menshikov, M. Y. (2016) Regulation of
adipose tissue stem cells angiogenic potential by tumor necrosis
factor-alpha, J. Cell. Biochem., 117, 180-196.
108.Efimenko, A., Starostina, E., Kalinina, N., and
Stolzing, A. (2011) Angiogenic properties of aged adipose derived
mesenchymal stem cells after hypoxic conditioning, J. Transl.
Med., 9, 10.
109.Aleksandrushkina, N. A., Danilova, N. V.,
Grigorieva, O. A., Mal’kov, P. G., Popov, V. S., Efimenko, A. Y.,
and Makarevich, P. I. (2019) Cell sheets of mesenchymal stromal cells
effectively stimulate healing of deep soft tissue defects, Bull.
Exp. Biol. Med., 167, 159-163.
110.Baertschiger, R. M., Serre-Beinier, V., Morel,
P., Bosco, D., Peyrou, M., Clement, S., Sgroi, A., Kaelin, A., Buhler,
L. H., and Gonelle-Gispert, C. (2009) Fibrogenic potential of human
multipotent mesenchymal stromal cells in injured liver, PLoS
One, 4, e6657.
111.Shi, S., and Gronthos, S. (2003) Perivascular
niche of postnatal mesenchymal stem cells in human bone marrow and
dental pulp, J. Bone Miner. Res., 18, 696-704.
112.Murphy, A. R., Laslett, A., O’Brien, C.
M., and Cameron, N. R. (2017) Scaffolds for 3D in vitro culture
of neural lineage cells, Acta Biomater., 54, 1-20.
113.Pourquie, O., Al Tanoury, Z., and Chal, J.
(2018) The long road to making muscle in vitro, Curr. Top.
Dev. Biol., 129, 123-142.
114.Syverud, B. C., VanDusen, K. W., and Larkin, L.
M. (2016) Growth factors for skeletal muscle tissue engineering,
Cells Tissues Organs, 202, 169-179.
115.Cosson, S., Otte, E. A., Hezaveh, H., and
Cooper-White, J. J. (2015) Concise review: tailoring bioengineered
scaffolds for stem cell applications in tissue engineering and
regenerative medicine, Stem Cells Transl. Med., 4,
156-164.
116.Lane, S. W., Williams, D. A., and Watt, F. M.
(2014) Modulating the stem cell niche for tissue regeneration, Nat.
Biotechnol., 32, 795-803.
117.Efimenko, A. Y., Kochegura, T. N., Akopyan, Z.
A., and Parfyonova, Y. V. (2015) Autologous stem cell therapy: how
aging and chronic diseases affect stem and progenitor cells, Biores.
Open Access, 4, 26-38.
118.Chacon-Martinez, C. A., Koester, J., and
Wickstrom, S. A. (2018) Signaling in the stem cell niche: regulating
cell fate, function and plasticity, Development, 145.
119.Mohamed, T. M. A., Ang, Y. S., Radzinsky, E.,
Zhou, P., Huang, Y., Elfenbein, A., Foley, A., Magnitsky, S., and
Srivastava, D. (2018) Regulation of cell cycle to stimulate adult
cardiomyocyte proliferation and cardiac regeneration, Cell,
173, 104-116e12.
120.Huang, P., Zhang, L., Gao, Y., He, Z., Yao, D.,
Wu, Z., Cen, J., Chen, X., Liu, C., Hu, Y., Lai, D., Hu, Z., Chen, L.,
Zhang, Y., Cheng, X., Ma, X., Pan, G., Wang, X., and Hui, L. (2014)
Direct reprogramming of human fibroblasts to functional and expandable
hepatocytes, Cell Stem Cell, 14, 370-384.