Neurotrophins of the Fetal Brain and Placenta in Prenatal Hyperhomocysteinemia
A. V. Arutjunyan1,a*, Yu. P. Milyutina1, A. D. Shcherbitskaia2, G. O. Kerkeshko1, I. V. Zalozniaia1, and A. V. Mikhel1
1Ott Institute of Obstetrics, Gynecology, and Reproductology, 199034 St. Petersburg, Russia2Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences, 194223 St. Petersburg, Russia
* To whom correspondence should be addressed.
Received September 25, 2019; Revised October 28, 2019; Accepted November 11, 2019
Prenatal hyperhomocysteinemia (PHHC) in pregnant rats was induced by chronic L-methionine loading, resulting in a significant increase in the L-homocysteine content both in the mothers’ blood and blood and brain of fetuses. Significant decrease in the weight of the placenta, fetus, and fetal brain was detected by the morphometric studies on day 20 of pregnancy. PHHC also activated maternal immune system due to the increase in the content of proinflammatory interleukin-1β in the rat blood and fetal part of the placenta. PHHC elevated the levels of the brain-derived neurotrophic factor (BDNF, 29 kDa) and nerve growth factor (NGF, 31 kDa) precursors in the placenta and the content of the BDNF isoform (29 kDa) in the fetal brain. The content of neuregulin 1 (NRG1) decreased in the placenta and increased in the fetal brain on day 20 of embryonic development. An increase in the caspase-3 activity was detected in the brains of fetuses subjected to PHHC. It was suggested that changes in the processing of neurotrophins induced by PPHC, oxidative stress, and inflammatory processes initiated by it, as well as apoptosis, play an important role in the development of brain disorders in the offspring.
KEY WORDS: prenatal hyperhomocysteinemia, neurotrophic factors, proinflammatory cytokines, placenta, fetus brainDOI: 10.1134/S000629792002008X
Abbreviations: BDNF, brain-derived neurotrophic factor; E3, E7, E12, and E20, days 3, 7, 12, and 20 of embryonic development; FPP, fetal part of placenta; HC, homocysteine; HHC, hyperhomocysteinemia; IL-1β, interleukin-1β; IL-6, interleukin-6; MPP, maternal part of placenta; NGF, nerve growth factor; NRG1, neuregulin 1; p75NTR, p75 neurotrophin receptor; PHHC, prenatal hyperhomocysteinemia; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α.
Prenatal hyperhomocysteinemia (PHHC) is one of the factors that causes
changes in the structure and function of cells, organ systems, and
homeostasis pathways in a developing organism and increases the risk of
development of various pathological conditions. Despite the fact that
placenta plays a key role in the adaptation of maternal organism to
pregnancy and fetus development, little is known about the processes
that characterize its functional state and can be affected by
neurotoxic compounds. Such compounds can disrupt the development of
fetal nervous system in the early neonatal period, often resulting in
the emergence of severe neurological and psychiatric disorders in
children and adults (autism, schizophrenia) [1-3].
Recently, the number of studies on the placental neurotrophic factors participating in the formation of the fetal nervous system has considerably increased. It is known that the brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) participate in the vital processes of neuronal growth and differentiation in the central and peripheral nervous systems of a developing fetus [4-7]. Another group of proteins involved in the neuronal development that has attracted attention of researchers is neuregulins that perform the protective function during fetus development and in newborns [8, 9].
Disruption of fetal brain functions by homocysteine (HC) could be associated with the increase in the concentration of proinflammatory cytokines in the maternal blood. These factors can cross the placental barrier and cause long-term impairments in the brain development in the offspring. The effect of proinflammatory cytokines is usually mediated via changes in the content of neurotrophic factors not only in the fetal brain, but also in the placenta, where these factors presumably exhibit the cytoprotective effect [4-7].
The objective of our study was investigation of the PHHC development induced by chronic methionine loading in rats and elucidation of the PHHC effect on the content of proinflammatory cytokines and neurotrophic compounds, such as BDNF, NGF, and neuregulin 1 (NRG1), essential for the fetal nervous system development, in the placenta and fetal brain.
MATERIALS AND METHODS
Pregnant Wistar rats (5-6-month-old) were divided into two groups. Animals of the first (control) group were fed with a standard chow. The second group received methionine loading in addition to the standard chow using a previously developed technique for modeling maternal hyperhomocysteinemia (HHC) that involved peroral administration of methionine by force-feeding animals with 0.15% aqueous L-methionine solution (0.10-0.15 g per animal on the 4th day of pregnancy, E4) [10]. The HHC state was evaluated from the total amount of L-HC in the rat blood serum 1, 6, 18, and 24 h after methionine administration using an Architect i1000 immunochemical analyzer (Abbott, USA). In order to monitor the daily dynamics of HC concentration in the blood, blood samples were collected on days E3 (i.e., prior to methionine administration), E7, and E12 from the gums [11], and after decapitation on E20. On E20, the rats were decapitated, and fetuses and placentas were removed from female rats in both groups. Placentas and blood serum from the mothers and entire brains from the fetuses were analyzed. Fetal brain tissue and blood were also sampled after the same time periods following administration of methionine or water. Typical developmental parameters such as placenta weight, fetus body weight, and fetus brain weight on E20 were determined. Blood serum was separated by centrifugation (2000g, 10 min) and stored at –80°C. Before analysis, the blood was washed away from the placenta with 0.001 M phosphate buffer (pH 7.4), and placenta was separated into the maternal (MPP) and fetal (FPP) parts.
The content of proinflammatory cytokines interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α) in the maternal blood serum and supernatants of placenta and fetal brain was determined by ELISA using Quantikine Rat IL-6, Quantikine Rat IL-1 beta/IL-1F2, and Quantikine Rat TNF-α kits (R&D Systems, USA), respectively.
The content of NRG1 in the placenta and fetal brain was determined using NRG1-beta 1 ELISA Kit (RayBiotech, USA) according to the manufacturer’s protocol.
The homogenates of brain and placenta tissues for Western blot analysis were prepared in 0.001 M phosphate buffer (pH 7.4) at a ratio 1 : 2 (w/v) in a glass homogenizer and centrifuged at 16,000g for 20 min to remove the cell debris. Total protein concentration in the samples was evaluated with the Bradford protein assay [12]. The samples (50 μg of protein) were separated in a 10% polyacrylamide gel under denaturing conditions according to Laemmli and transferred onto a PVDF membrane. The membranes were blocked with 2% albumin (Sigma-Aldrich, USA) in TBST (50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20). BDNF was probed with rabbit anti-BDNF antibody [EPR1292] (1 : 1000; Abcam, USA); relative NGF concentration was evaluated with rabbit anti-NGF antibody [EP1320Y] (1 : 1000; Abcam) that recognized immunoreactive ~30 kDa NGF forms. Following incubation with the horse-radish peroxidase (HRP) conjugates of goat antibodies (1 : 1000; BioRad, USA), the signal was visualized using enhanced chemiluminescence (ECL; BioRad). The intensities of obtained protein bands were evaluated with the ImageLab program. Based on the existing recommendations for normalization of the target protein content [13], the obtained data were normalized to the content of glyceraldehyde-3-phosphate dehydrogenase [GAPDH (14C10) rabbit antibody, 1 : 1000; Cell Signaling, USA]; total protein content in the gel was determined using the stain-free technology (BioRad) according to the manufacturer’s instruction.
Caspase-3 activity was assayed in 20 mM HEPES, containing 0.1% CHAPS, 2 mM EDTA, and 5 mM DTT (pH 7.4) using 4 mM synthetic peptide Ac-DEVD-pNA (N-acetyl-Asp-Glu-Val-Asp p-nitroanilide) as a substrate. Samples containing 120 μg of protein were incubated at 37°C for 10 min; the reaction was initiated by adding the substrate, and the absorbance of the reaction mixture was recorded at 405 nm at 37°C every 5 min for 25 min. The activity of caspase-3 was expressed as micromoles of the reaction product pNA/min per mg protein.
The data obtained were processed with the STATISTICA 10.0 (StatSoft, USA). To compare the results, the non-parametric Mann–Whitney U-test or parametric t-test for random samples was used. Data distribution was evaluated with the Shapiro–Wilk test. The data are presented as mean ± SEM or Me [25%, 75%] (Me, median; 25% and 75%, first and third quartile, respectively) for at least six independent experiments. Significance level was set at 0.05.
RESULTS
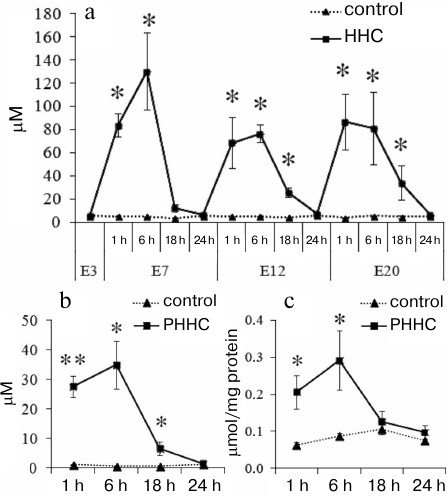
Methionine loading increased the HC level in the blood of pregnant rats and in the blood and brain of fetuses (Fig. 1). On day E3, prior to the administration of water (control) or methionine (experimental group), the HC content in the bloof of female rats was 5.70 ± 0.37 μM in the control group and 5.70 ± 0.70 μM in the experimental group. On day E7, the blood from the female rats was sampled 1, 6, 18, and 24 h after methionine administration. As can be seen in Fig. 1a, a significant (p ≤ 0.01) increase in the HC content was observed 1 h after per oral methionine administration with the maximal HC concentration (130.60 ± 33.48 μM) after 6 h vs. the control group of animals (5.23 ± 0.10 μM). However, after 18 h, the HC content in the experimental group decreased to the concentration observed in the control group (5.46 ± 0.14 μM). On days E12 and E20, the dynamics of changes in the HC content was the same, but the HC concentration in experimental group decreased to the normal level significantly later (24 h after methionine administration). The maximal level of HC that exceeded the control more than 10 times was observed 1-6 h after methionine administration.
Fig. 1. Daily dynamics of HC content 1, 6, 18, and 24 h after administration of methionine (HHC) or water (control) in the blood serum of (a) pregnant female rats on days E3, E7, E12, and E20 (n = 5-7) and (b) fetuses on day E20 (n = 4-6) (PHHC and control). c) Daily dynamics of HC content in the brain of fetuses on day E20 (n = 4-6) (PHHC, control); * p ≤ 0.05, ** p ≤ 0.01. The data are presented as mean ± standard error of the mean (M ± SEM).
Chronic administration of methionine to pregnant rats caused the increase in the HC level after each methionine load not only in the maternal blood, but also in the blood and brain of fetuses on day E20. The HC level rapidly reached it maximum after 1-6 h, after which it gradually decreased within 24 h to the level statistically undistinguishable from the control (Fig. 1, b and c).
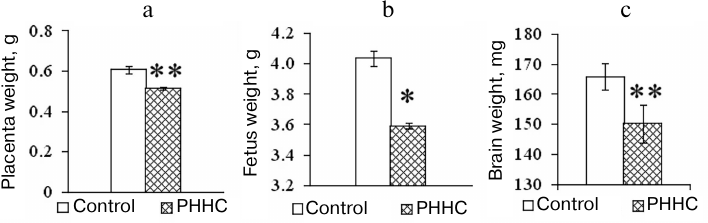
In addition to the increase in the HC content in the blood, changes in the morphometric parameters were observed in the animals with PHHC. Thus, a decrease in the placenta weight and fetus weight was found in the PHHC animals on day E20 (p < 0.001). It should be emphasized that the brain mass of fetuses subjected to PHHC decreased by almost 10% vs. control (Fig. 2).
Fig. 2. The weight of placenta (n = 80) (a), fetus (n = 80) (b), and fetal brain (n = 80) (c) on day E20 in the control and experimental groups; * p ≤ 0.05, ** p ≤ 0.01. The data are presented as mean ± standard error of the mean (M ± SEM).
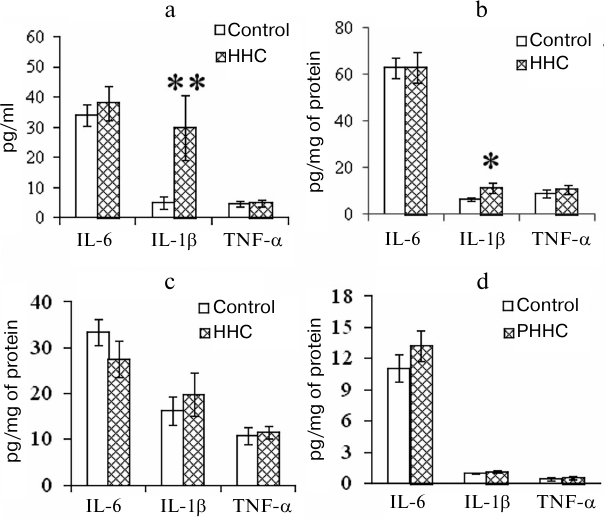
PHHC also affected the levels of proinflammatory cytokines in the test animals. The concentration of IL-1β increased in the blood of pregnant rats and FPP 24 h after the last administration of methionine. As shown in Fig. 3, a and b, the content of IL-1β in the serum of PHHC animals increased 6-fold and reached 30 pg/ml (p < 0.001); in the FPP, it increased approximately 2-fold and reached >10 pg/mg of protein (p < 0.05). The level of proinflammatory cytokines in the MPP did not change (Fig. 3c). HHC produced no effect on the concentration of IL-6 and TNF-α in the maternal blood and placenta. No changes were observed in the content of IL-6, TNF-α, and IL-1β in the fetal brain on day E20 (Fig. 3d).
Fig. 3. The content of proinflammatory cytokines in the (a) blood serum of pregnant female rats (n = 13), (b) FPP (n = 13), (c) MPP (n = 13), and (d) fetal brain on day E20 in the control group and rats with PHHC. The data are presented as mean ± standard error of the mean (M ± SEM); * p ≤ 0.05, ** p ≤ 0.01.
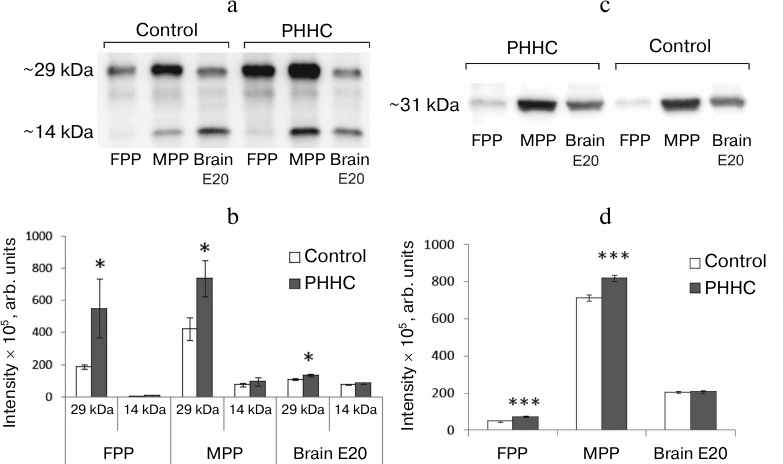
According to the Western blotting data, MPP, FPP, and fetal brain samples on day E20 demonstrated two protein bands with molecular masses of ~14 kDa corresponding to the mature BDNF (mBDNF) and ~29 kDa corresponding to the BDNF precursor (proBDNF) and/or its truncated form (truncated BDNF) (Fig. 4, a and b). In the FPP, mBDNF was present in trace amounts, while in the MPP, its amount was similar to that in the fetal brain (75.60 ± 11.37 and 78.50 ± 3.22 arb. units, respectively) and was not affected by PHHC. On the other hand, the content of 29 kDa BDNF isoform in FPP and MPP was significantly higher in comparison with mBDNF and increased in PHHC (p < 0.05). The amount of this isoform in the fetal brain was also higher than the content of mBDNF and increased in PHHC. In placenta, the amount of mBDNF was significantly lower than the content of the 29 kDa isoform (p14/p29 in ratio in the MPP in the control group was 0.195 ± 0.027 vs. 0.130 ± 0.029 in the experimental group; p14/p29 ratio in the FPP in the control group was 0.017 ± 0.014 vs. 0.011 ± 0.005 in the experimental group), while in the fetal brain, both proteins were present in similar quantities (p14/p29 ratio in the control group was 0.707 ± 0.035 vs. 0.668 ± 0.037 in the PHHC group). PHHC upregulated the content of the 29 kDa isoform in both placenta and fetal brain but did not affect the amount of the 14 kDa isoform. The content of the 29 kDa isoform increased to the largest degree in the MPP. The data presented in Fig. 4, c and d, demonstrate that immunoblotting could be used successfully not only for detection of BDNF isoforms, but also for NGF identification in the placenta. It was established MPP, FPP, and fetal brain on day E20 contained NGF isoform with a molecular mass ~31 kDa. The NGF content increased noticeably in PHHC, except in the fetal brain, where only a trend for its increase was observed, which was likely due to the wide scattering of the obtained data.
Fig. 4. BDNF and NGF content in the FPP (n = 6), MPP (n = 10), and fetal brain (n = 10) on day E20 in the control and PHHC rats. a) Representative Western blot of two BDNF isoforms (~29 and ~14 kDa). b) The content of BDNF isoforms (y-axis, band intensity determined in pixels and expressed in arbitrary units). c) Representative Western blot of NGF. d) The content of NGF (y-axis, band intensity determined in pixels and expressed in arbitrary units). The data are presented as mean ± standard error of the mean (M ± SEM); *** p ≤ 0.01; * p ≤ 0.05.
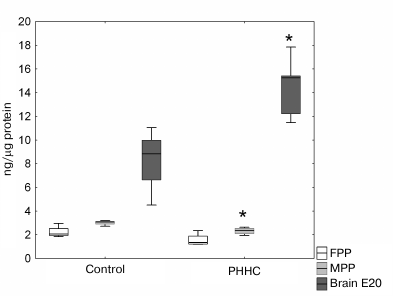
No reliable difference in the NRG1 content in the FPP was observed between the experimental and control groups. However, a significant decrease in the content of this growth factor in the MPP was found in the PHHC group (p < 0.05). In the fetal brain on day E20, the NRG1 content in this group was 1.66 times higher compared to the control group (Fig. 5).
Fig. 5. NRG1 content in the FPP, MPP, and fetal brain on day E20 in the control and PHHC rats evaluated by ELISA (n = 8). Data presented as Me [25%, 75%]; * p ≤ 0.05.
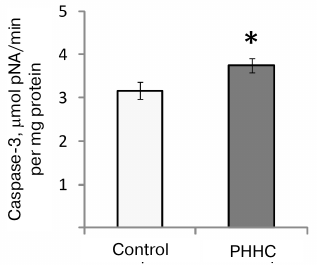
An increase in the caspase-3 activity (evaluated as an increased rate of reaction product accumulation) was observed in the fetal brain on day E20 in the PHHC group (3.74 ± 0.17 μmol pNA/min per mg protein vs. 3.16 ± 0.19 μmol pNA/min per mg protein in the control group; p < 0.05) (Fig. 6).
Fig. 6. Caspase-3 activity in the fetal brain homogenate on day E20 in the control (n = 16) and PHHC (n = 21) rats. Data are presented as mean ± standard error of the mean (M ± SEM); * p ≤ 0.05.
DISCUSSION
Despite the fact that PHHC results in the development of severe functional central nervous system disorders in the offspring, as it has been demonstrated in our previous studies [14, 15] and by other research groups [16-21], it still remains unclear, what causes these disruptions and to what degree they are mediated by changes in the functional state of the placenta. The cited studies have revealed that maternal HHC is accompanied by the increase in the HC content in the blood of newborn animals. It is important to mention that in this study, we for the first time detected PHHC in fetuses from mothers that had elevated HC level during gestation. It can be assumed that the increased amounts of HC formed due to the disruption of its metabolism can easily cross the placental barrier via simple diffusion or by binding to specific transporters [22]. We have established previously that one of the causes of the PHHC neurotoxic effect was an increased sensitivity of the nervous system cells to excitotoxic and oxidative damage, which was manifested as suppression of the functioning of glutamate NMDA receptors under these conditions [23], decrease in the neuron survival rate observed upon the elevated generation of reactive oxygen species (ROS), decrease in the content of low-molecular-weight antioxidants, and inhibition of the antioxidant enzymes [24, 25]. The role of oxidative stress in the HHC-associated disruption of the nervous system development and cognitive functions in the offspring was also corroborated by the observations that the consequences of HHC can be alleviated by administration of melatonin and certain short peptides with the antioxidant properties to the animals during gestation [14, 17, 26].
Our results on the stimulation of IL-1β production during oxidative stress in PHHC are in agreement with the experimental data produced in mature animals by other researchers. It was established that both acute and chronic HHC causes increase in the concentration of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in the rat blood and brain [27, 28]. It was suggested that one of the mechanisms of the proinflammatory effect of HC is its impact on placental macrophages, which results in the upregulation of synthesis and secretion of proinflammatory cytokines [29]. An increased level of ROS generation by the macrophages and vascular endothelium in response to the elevated blood levels of HC plays an important role in activation of cytokine secretion. HHC can cause systemic inflammation in endothelial cells via enhanced ROS generation, which is accompanied by the release of IL-1β [30]. The obtained results indicate that PHHC causes excessive activation of maternal immune system, resulting in the increased blood levels of proinflammatory cytokines, such as IL-1β, that can cross the fetoplacental barrier and cause neurodegeneration and other long-term disorders in the brain of the offspring [31-33]. An increased content of IL-1β could be due to the induction of its expression facilitated by the glutamate NMDA receptors, as it was demonstrated in a number of studies on focal brain ischemia [34, 35]. Based on the significance of NMDA receptor signaling in the HC neurotoxicity, its involvement in the development of PHHC-associated conditions cannot be excluded.
Similar effect of IL-1β and other proinflammatory cytokines could be mediated by changes in the content of neurotrophic factors induced by these cytokines both in the fetal brain and in the placenta, where these factors supposedly exhibit the cytoprotective effect and play an important role in placental angiogenesis [36-38]. The data obtained indicate significant changes in the neurotrophin levels in the placenta. It is known that some neuroactive factors are synthesized in the placenta, cross the fetoplacental barrier, and exert their effect on the fetal brain development [36, 39]. Moreover, it can be expected that production of these compounds could alter during the development of proinflammatory process induced by PHHC. In this regard, it is commonly believed that neurotrophins (including BDNF and NGF investigated in this study) produced by various immune cells play an essential role in the development of neurodegenerative disorders [40].
NRG1 (together with BDNF and NGF) participates in the regulation of cells proliferation, migration, differentiation, and synaptogenesis. As was demonstrated in this work, the content of NRG1 increased in the fetal brain on day E20 in parallel to its decrease in the MPP. NRG1 also participates in the regulation of methionine metabolism (and hence, HC metabolism) in neurons via stimulation of methionine synthase activity by methylcobalamin and activation of glutathione synthesis [41]. Our results suggest that placental NRG1 can protect the fetus against the neurotoxic effect of maternal HHC. Despite the established role of NRG1 in the development of fetal brain [8, 9], there are only few studies on the functions of this factor in the placenta. NRG1 is expressed and secreted by decidual stromal cells; it was suggested that it plays a role in the paracrine regulation of cell survival and differentiation, as well as invasion of the extravillous trophoblast cells, via activation of signaling pathways leading to the apoptosis suppression [42]. It is well established that HHC promotes apoptosis, resulting in the death of trophoblasts [43, 44].
The concentration of neurotrophic factors BDNF and NGF in the placenta increased in PHHC. Both BDNF and NGF exhibit the antiapoptotic properties [45]. However, this increase was observed only for the precursors of these factors. The content of mBDNF formed as a result of proteolytic processing of the precursor protein proBDNF [46, 47] in the placenta was insignificant and remained unaffected by PHHC. Some authors suggest that the critical periods in development of placenta and fetal brain are influenced by similar biological signals [48]. The fact that BDNF is one of the factors actively participating in the development of fetal nervous system implies parallel changes in the BDNF content in the fetal brain and placenta at the same stages of prenatal development [49]. Our data on the comparable levels of neurotrophins in the developing brain (E20) and placenta are in agreement with the results of studies reporting that expression of BDNF mRNA in rat placenta increases in gestation time and reaches maximum on E21 [5]. It was established that proBDNF exhibits the opposite to mBDNF effect on the survival and functioning of neurons and synaptic plasticity development [50], and this effect is most pronounced in neurodegenerative pathologies [51]. Unlike mBDNF, which interacts with the tyrosine kinase receptor (Trk-B) of tropomyosin with a high affinity, proBDNF binds to the neurotrophin receptor p75 (p75NTR), the activation of which results in the suppression of neuronal proliferation and activation of apoptosis [52]. Furthermore, it was suggested that proBDNF is a neurotrophin present predominantly during the early prenatal period, while mBDNF manifests its effects mainly in the adult organism [53, 54].
Commonly used enzyme immunoassays do not allow to identify different molecular isoforms of BDNF and other neurotrophins. BDNF is synthesized as its precursor pre-proBDNF with the molecular mass of 35 kDa that is then cleaved with the formation of proBDNF (32 kDa). The following proteolysis of proBDNF by intracellular proteolytic enzymes generates mBDNF with the molecular mass of 13-15 kDa [55-57] and/or the so-called truncated form (28 kDa) with yet unknown function [58, 59]. NGF and BDNF are expressed differently in different regions of the placenta [60]. We found that the content of studied neurotrophins in the MPP and FPP differed significantly; in particular, higher BDNF and NGF concentrations and, especially, high mBDNF to proBDNF ratio were observed in the MPP. Considering that the functions of BDNF isoforms in the developing brain and different regions of placenta could vary, some researchers believe that it is more informative to measure also the mBDNF to proBDNF ratio than the levels of individual of BDNF isoforms alone [61]. The dominance of proBDNF, reduced content of mBDNF, and, hence, lower mBDNF to proBDNF ratio in the MPP and especially in the FPP compared to the fetal brain on day E20 were demonstrated by us in this study for the first time. These differences could be due to either a lower rate of pro-neurotrophin processing into its mature form and/or fast degradation of mBDNF in the placenta in comparison with the fetal brain.
Analysis of the published data suggests that the dynamic balance between all BDNF isoforms (including truncated form) generated during the processing plays an important role in the plasticity of the nervous system and disruption of cognitive functions in different pathologies [58].
NGF processing also includes proteolytic cleavage of precursors to mature forms exhibiting high affinity to the tropomyosin Trk kinase receptors, the binding to which underlies the neuroprotective effect of NGF and survival of nerve cells. Interaction of proNGF and proBDNF with the p75NTR receptor initiates apoptosis and exerts the neurotoxic effect [62, 63] on both neurons and glial cells by stimulating inflammatory processes due to the upregulated production of TNF-α [64]. Using immunoblotting, we demonstrated that in the rodent brain, NGF was almost entirely represented by the precursor with the molecular mass of 32 kDa [63], while mNGF was virtually undetectable because of its negligible content [65]. Such low content of mNGF could be due to the fact that proNGF easily forms a stable complex with soluble tissue alpha-2-macroglobulin, which increases its resistance to proteolytic cleavage [62]. It was also suggested that secreted NGF pool contains both forms (proNGF and mNGF) [63], while ELISA allows to detect only the net content of this neurotrophin.
According to the results obtained in this work, PHHC causes an increase in the content of BDNF and NGF precursors in the placenta, which might be due to their slower processing and results in the impaired fetus development. Based on the analysis of published data, BDNF and NGF isoforms with molecular masses of 29 and 31 kDa, respectively, identified in our study are most likely precursors of neurotrophin mature forms with the neurotrophic properties. One cannot rule out that the 29 kDa band could be the truncated form of BDNF. According to some researchers, the difference between the HHC-induced changes in the neurotrophin concentrations in the MPP and FPP could be explained by different levels of oxidative stress evolving under pathological conditions in different parts of the placenta. In turn, this causes compensatory response manifested by the increase in the concentration of neurotrophins, which are required for the formation of viable fetus in pathological pregnancy and under stress conditions [66]. The data on the PHHC impact on the amount of neurotrophin precursors in the placenta were corroborated by the data obtained for the fetal brain on day E20 that revealed an increased formation of neurotrophin precursors in HHC. This increase was statistically significant for BDNF and was observed as a trend for NGF. The content of mBDNF in the placenta and fetal brain was not affected by PHHC.
Several mechanisms could be suggested to explain the negative effect of PHHC on the developing fetus. The HC toxicity discovered in this work results in the decrease of such vital parameters as placenta weight, fetus weight, and fetus brain mass. One of the factors causing these consequences could be the HHC-induced oxidative stress, as reported in the studies of our [25] and other research groups [14, 17, 19]. Oxidative stress development is facilitated by the inflammatory response stimulation by PHHC via enhanced formation of IL-1β in the FPP and blood of pregnant females. It is known that one of the mechanisms underlying the damaging effect of oxidative stress is apoptosis induced by the elevated ROS generation and following cell death in both placenta and fetal brain. The results on the oxidative stress development in the brain of newborns subjected to PHHC obtained in [25] and the data from this study on the increase in the activity of caspase-3 (key cysteine protease of the apoptotic cascade) in the fetal brain are in agreement with the modern concepts on the mechanisms underlying the damaging effect of HC, in particular, apoptosis development upon the oxidative stress intensification. According to the published data, apoptosis activation during HHC in different types of cells could occur via either the external pathway through the action of extracellular signal on the cell surface receptors or internal pathway associated with mitochondria destruction under oxidative stress. It was observed for HC-exposed trophoblast [43, 44] and brain cells of the offspring of rats subjected to PHHC [16].
Another mechanism that can play a significant role in the damaging action of HHC is epigenetic modification via methylation (primarily, DNA methylation), because formation of S-adenosyl-HC from S-adenosyl methionine (main donor of methyl groups) results in the inhibition of methylation processes in the organism [60]. Thus, it was found that folate deficiency in HHC decreases the level of DNA methylation in the placenta, which impairs fetal growth and development [67, 68]. It is commonly recognized that DNA methylation plays an important role in the regulation of expression of neurotrophin genes, BDNF in particular, during the nervous system development and in a number of neurodegenerative diseases [67, 68]. In this regard, studying the relationship between the processing of various forms of neurotrophins and extent of DNA methylation is of particular interest for undestanding the factors disrupting placenta functioning and fetal development in PHHC.
In conclusion, we observed significant changes in the morphometric parameters, IL-1β levels, and content of neurotrophic factors (BDNF, NGF, NRG1) in the placenta and disruption of the fetal brain development in experimental PHHC caused by chronic methionine loading in pregnant rats. The data obtained open the possibilities for clinical investigation of the indicated parameters in the placenta as markers of unfavorable changes in the formation of nervous system in newborns in HHC, which is one of the common complications of pregnancy.
Funding. This work was supported by the Russian Foundation for Basic Research (project 18-015-00099) and by the State Budget Project no. AAAA-A19-119021290116-1.
Conflict of interest. The authors declare no conflict of interest in financial or any other sphere.
Ethical approval. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
1.Onore, C., Careaga, M., and Ashwood, P. (2012) The
role of immune dysfunction in the pathophysiology of autism, Brain
Behav. Immun., 26, 383-392; doi:
10.1016/j.bbi.2011.08.007.
2.Patterson, P. H. (2009) Immune involvement in
schizophrenia and autism: etiology, pathology and animal models,
Behav. Brain Res., 204, 313-321; doi:
10.1016/j.bbr.2008.12.016.
3.Wahlbeck, K., Forsen, T., Osmond, C., Barker, D.
J., and Eriksson, J. G. (2001) Association of schizophrenia with low
maternal body mass index, small size at birth, and thinness during
childhood, Arch. Gen. Psychiatry, 58, 48-52; doi:
10.1001/archpsyc.58.1.48.
4.Dhobale, M. V., Pisal, H. R., Mehendale, S. S., and
Joshi, S. R. (2013) Differential expression of human placental
neurotrophic factors in preterm and term deliveries, Int. J. Dev.
Neurosci., 31, 719-723; doi:
10.1016/j.ijdevneu.2013.09.006.
5.Garces, M. F., Sanchez, E., Torres-Sierra, A. L.,
Ruiz-Parra, A. I., Angel-Muller, E., Alzate, J. P., Sanchez, A. Y.,
Gomez, M. A., Romero, X. C., Castaneda, Z. E., Sanchez-Rebordelo, E.,
Dieguez, C., Nogueiras, R., and Caminos, J. E. (2014) Brain-derived
neurotrophic factor is expressed in rat and human placenta and its
serum levels are similarly regulated throughout pregnancy in both
species, Clin. Endocrinol. (Oxf.), 81, 141-151; doi:
10.1111/cen.12391.
6.Tapia-Arancibia, L., Rage, F., Givalois, L., and
Arancibia, S. (2004) Physiology of BDNF: focus on hypothalamic
function, Front. Neuroendocrinol., 25, 77-107; doi:
10.1016/j.yfrne.2004.04.001.
7.Tometten, M., Blois, S., and Arck, P. C. (2005)
Nerve growth factor in reproductive biology: link between the immune,
endocrine and nervous system? Chem. Immunol. Allergy, 89,
135-148; doi: 10.1159/000087962.
8.Dammann, O., Bueter, W., Leviton, A., Gressens, P.,
and Dammann, C. E. (2008) Neuregulin-1: a potential endogenous
protector in perinatal brain white matter damage, Neonatology,
93, 182-187; doi: 10.1159/000111119.
9.Esper, R. M., Pankonin, M. S., and Loeb, J. A.
(2006) Neuregulins: versatile growth and differentiation factors in
nervous system development and human disease, Brain Res. Rev.,
51, 161-175; doi: 10.1016/j.brainresrev.2005.11.006.
10.Arytjunyan, A. V., Milyutina, Yu. P., Zaloznyaya,
I. V., Pustygina, A. V., Kozina, L. S., and Korenevskii, A. V. (2012)
Use of different experimental models of hyperhomocysteinemia in
neurochemical studies, Neurochem. J., 6, 71-76; doi:
10.1134/S1819712411040027.
11.Zil’fyan, V. N., and Kumkumadzhyan, V. A.
(1970) New technique for sampling blood from small laboratory animals,
Zh. Eksp. Klin. Med., 10, 12-14.
12.Bradford, M. M. (1976) A rapid and sensitive
method for the quantitation of microgram quantities of protein
utilizing the principle of protein–dye binding, Anal.
Biochem., 72, 248-254; doi: 10.1006/abio.1976.9999.
13.Bass, J. J., Wilkinson, D. J., Rankin, D.,
Phillips, B. E., Szewczyk, N. J., Smith, K., and Atherton, P. J. (2017)
An overview of technical considerations for Western blotting
applications to physiological research, Scand. J. Med. Sci.
Sports, 27, 4-25; doi: 10.1111/sms.12702.
14.Arutjunyan, A., Kozina, L., Stvolinskiy, S.,
Bulygina, Y., Mashkina, A., and Khavinson, V. (2012) Pinealon protects
the rat offspring from prenatal hyperhomocysteinemia, Int. J. Clin.
Exp. Med., 5, 179-185.
15.Shcherbitskaya, A. D., Milyutina, Y. P.,
Zaloznyaya, I. V., Arutjunyan, A. V., Nalivaeva, N. N., and Zhuravin,
I. A. (2017) The effects of prenatal hyperhomocysteinemia on the
formation of memory and the contents of biogenic amines in the rat
hippocampus, Neurochem. J., 11, 296-301; doi:
10.1134/s1819712417040080.
16.Koz, S. T., Gouwy, N. T., Demir, N., Nedzvetsky,
V. S., Etem, E., and Baydas, G. (2010) Effects of maternal
hyperhomocysteinemia induced by methionine intake on oxidative stress
and apoptosis in pup rat brain, Int. J. Dev. Neurosci.,
28, 325-329; doi: 10.1016/j.ijdevneu.2010.02.006.
17.Baydas, G., Koz, S. T., Tuzcu, M., and
Nedzvetsky, V. S. (2008) Melatonin prevents gestational
hyperhomocysteinemia-associated alterations in neurobehavioral
developments in rats, J. Pineal Res., 44, 181-188; doi:
10.1111/j.1600-079X.2007.00506.x.
18.Baydas, G., Koz, S. T., Tuzcu, M., Nedzvetsky, V.
S., and Etem, E. (2007) Effects of maternal hyperhomocysteinemia
induced by high methionine diet on the learning and memory performance
in offspring, Int. J. Dev. Neurosci., 25, 133-139; doi:
10.1016/j.ijdevneu.2007.03.001.
19.Makhro, A. V., Mashkina, A. P., Solenaya, O. A.,
Trunova, O. A., Kozina, L. S., Arutjunyan, A. V., and Bulygina, E. R.
(2008) Prenatal hyperhomocysteinemia as a model of oxidative stress of
the brain, Bull. Exp. Biol. Med., 146, 33-35; doi:
10.1007/s10517-008-0233-0.
20.Gerasimova, E., Yakovleva, O., Burkhanova, G.,
Khaertdinov, N., Sitdikova, G., and Ziyatdinova, G. (2017) Effects of
maternal hyperhomocysteinemia on the early physical development and
neurobehavioral maturation of rat offspring, BioNanoScience,
7, 155-158; doi: 10.1007/s12668-016-0326-6.
21.Makhro, A. V., Mashkinba, A. P., Solenaya, O. A.,
Trunova, O. A., Tyulina, O. V., Bulygina, E. R., and Boldyrev, A. A.
(2008) Carnosine protects cells from oxidative stress induced by
hyperhomocysteinemia, Neurochem. J., 2, 202-208; doi:
10.1134/S1819712408030112.
22.Tsitsiou, E., Sibley, C. P., D’Souza, S.
W., Catanescu, O., Jacobsen, D. W., and Glazier, J. D. (2011)
Homocysteine is transported by the microvillous plasma membrane of
human placenta, J. Inherit. Metab. Dis., 34, 57-65; doi:
10.1007/s10545-010-9141-3.
23.Arutjunyan, A. V., Kozina, L. S., and Arutyunov,
V. A. (2010) Toxic effect of prtenataly hyperhomocysteinemia on
offsprings (experimental study), Zh. Akusher. Zhen. Bolez.,
59, 16-23.
24.Arutjunyan, A. V., Pustygina, A. V., Milyutina,
Yu. P., Zaloznyaya, I. V., and Kozina, L. S. (2015) Molecular markers
of oxidative stress in offsprings in experimental hyperhomocysteinemia,
Mol. Meditsina, 5, 41-46.
25.Pustygina, A. V., Milyutina, Yu. P., Zaloznyaya,
I. V., and Arutjunyan, A. V. (2015) Indices of oxidative stress in the
brain of newborn rats subjected to prenatal hyperhomocysteinemia,
Neurochem. J., 9, 60-65; doi:
10.1134/S1819712415010079.
26.Gitto, E., Pellegrino, S., Gitto, P., Barberi,
I., and Reiter, R. J. (2009) Oxidative stress of the newborn in the
pre- and postnatal period and the clinical utility of melatonin, J.
Pineal Res., 46, 128-139; doi:
10.1111/j.1600-079X.2008.00649.x.
27.Da Cunha, A. A., Ferreira, A. G., Loureiro, S.
O., da Cunha, M. J., Schmitz, F., Netto, C. A., and Wyse, A. T. (2012)
Chronic hyperhomocysteinemia increases inflammatory markers in
hippocampus and serum of rats, Neurochem. Res., 37,
1660-1669; doi: 10.1007/s11064-012-0769-2.
28.Da Cunha, A. A., Ferreira, A. G., and Wyse, A. T.
(2010) Increased inflammatory markers in brain and blood of rats
subjected to acute homocysteine administration, Metab. Brain
Dis., 25, 199-206; doi: 10.1007/s11011-010-9188-8.
29.Zanin, R. F., Bergamin, L. S., Morrone, F. B.,
Coutinho-Silva, R., de Souza Wyse, A. T., and Battastini, A. M. (2015)
Pathological concentrations of homocysteine increases IL-1beta
production in macrophages in a P2X7, NF-κB, and Erk-dependent
manner, Purinergic Signal, 11, 463-470; doi:
10.1007/s11302-015-9464-5.
30.Xu, X., Yang, X. Y., He, B. W., Yang, W. J., and
Cheng, W. W. (2016) Placental NRP1 and VEGF expression in pre-eclamptic
women and in a homocysteine-treated mouse model of pre-eclampsia,
Eur. J. Obstet. Gynecol. Reprod. Biol., 196, 69-75; doi:
10.1016/j.ejogrb.2015.11.017.
31.Bilbo, S. D., and Schwarz, J. M. (2009)
Early-life programming of later-life brain and behavior: a critical
role for the immune system, Front. Behav. Neurosci., 3;
doi: 10.3389/neuro.08.014.2009.
32.Jakubowski, H. (2004) Molecular basis of
homocysteine toxicity in humans, Cell. Mol. Life Sci.,
61, 470-487; doi: 10.1007/s00018-003-3204-7.
33.Smith, S. E., Li, J., Garbett, K., Mirnics, K.,
and Patterson, P. H. (2007) Maternal immune activation alters fetal
brain development through interleukin-6, J. Neurosci.,
27, 10695-10702; doi: 10.1523/JNEUROSCI.2178-07.2007.
34.Jander, S., Schroeter, M., and Stoll, G. (2000)
Role of NMDA receptor signaling in the regulation of inflammatory gene
expression after focal brain ischemia, J. Neuroimmunol.,
109, 181-187; doi: 10.1016/s0165-5728(00)00317-9.
35.Onufriev, M. V., Freiman, S. V., Moiseeva, Yu.
V., Stepanichev, M. Yu., Lazareva, N. A., and Gulyaeva, N. V. (2017)
Accumulation of corticosterone and interleukin-1 in the hippocampus
after focal ischemic damage of the neocortex: selective vulnerability
of the ventral hippocampus, Neurochem. J., 11,
236-241, doi: 10.1134/S1819712417030084.
36.Bolton, J. L., and Bilbo, S. D. (2014)
Developmental programming of brain and behavior by perinatal diet:
focus on inflammatory mechanisms, Dialogues Clin. Neurosci.,
16, 307-320.
37.Gilmore, J. H., Jarskog, L. F., and Vadlamudi, S.
(2003) Maternal infection regulates BDNF and NGF expression in fetal
and neonatal brain and maternal–fetal unit of the rat, J.
Neuroimmunol., 138, 49-55; doi:
10.1016/S0165-5728(03)00095-X.
38.Gilmore, J. H., Jarskog, L. F., and Vadlamudi, S.
(2005) Maternal poly I:C exposure during pregnancy regulates TNFalpha,
BDNF, and NGF expression in neonatal brain and the maternal–fetal
unit of the rat, J. Neuroimmunol., 159, 106-112; doi:
10.1016/j.jneuroim.2004.10.008.
39.Hsiao, E. Y., and Patterson, P. H. (2012)
Placental regulation of maternal–fetal interactions and brain
development, Dev. Neurobiol., 72, 1317-1326; doi:
10.1002/dneu.22045.
40.Vega, J. A., Garcia-Suarez, O., Hannestad, J.,
Perez-Perez, M., and Germana, A. (2003) Neurotrophins and the immune
system, J. Anat., 203, 1-19; doi:
10.1046/j.1469-7580.2003.00203.x.
41.Zhang, Y., Hodgson, N., Trivedi, M., and Deth, R.
(2016) Neuregulin 1 promotes glutathione-dependent neuronal cobalamin
metabolism by stimulating cysteine uptake, Oxid. Med. Cell.
Longev., 2016, 3849087; doi: 10.1155/2016/3849087.
42.Fock, V., Plessl, K., Draxler, P., Otti, G. R.,
Fiala, C., Knofler, M., and Pollheimer, J. (2015) Neuregulin-1-mediated
ErbB2-ErbB3 signalling protects human trophoblasts against apoptosis to
preserve differentiation, J. Cell Sci., 128, 4306-4316;
doi: 10.1242/jcs.176933.
43.Di Simone, N., Maggiano, N., Caliandro, D.,
Riccardi, P., Evangelista, A., Carducci, B., and Caruso, A. (2003)
Homocysteine induces trophoblast cell death with apoptotic features,
Biol. Reprod., 69, 1129-1134; doi:
10.1095/biolreprod.103.015800.
44.Kamudhamas, A., Pang, L., Smith, S. D., Sadovsky,
Y., and Nelson, D. M. (2004) Homocysteine thiolactone induces apoptosis
in cultured human trophoblasts: a mechanism for homocysteine-mediated
placental dysfunction? Am. J. Obstet. Gynecol., 191,
563-571; doi: 10.1016/j.ajog.2004.01.037.
45.Fujita, K., Tatsumi, K., Kondoh, E., Chigusa, Y.,
Mogami, H., Fujii, T., Yura, S., Kakui, K., and Konishi, I. (2011)
Differential expression and the anti-apoptotic effect of human
placental neurotrophins and their receptors, Placenta,
32, 737-744; doi: 10.1016/j.placenta.2011.07.001.
46.Yang, B., Ren, Q., Zhang, J. C., Chen, Q. X., and
Hashimoto, K. (2017) Altered expression of BDNF, BDNF pro-peptide and
their precursor proBDNF in brain and liver tissues from psychiatric
disorders: rethinking the brain–liver axis, Transl.
Psychiatry, 7, e1128; doi: 10.1038/tp.2017.95.
47.Hashimoto, K. (2016) Regulation of brain-derived
neurotrophic factor (BDNF) and its precursor proBDNF in the brain by
serotonin, Eur. Arch. Psychiatry Clin. Neurosci., 266,
195-197; doi: 10.1007/s00406-016-0682-9.
48.Zeltser, L. M., and Leibel, R. L. (2011) Roles of
the placenta in fetal brain development, Proc. Natl. Acad. Sci.
USA, 108, 15667-15668; doi: 10.1073/pnas.1112239108.
49.Saenen, N. D., Plusquin, M., Bijnens, E.,
Janssen, B. G., Gyselaers, W., Cox, B., Fierens, F., Molenberghs, G.,
Penders, J., Vrijens, K., De Boever, P., and Nawrot, T. S. (2015) In
utero fine particle air pollution and placental expression of genes
in the brain-derived neurotrophic factor signaling pathway: an
environage birth cohort study, Environ. Health Perspect.,
123, 834-840; doi: 10.1289/ehp.1408549.
50.Dincheva, I., Lynch, N. B., and Lee, F. S. (2016)
The role of BDNF in the development of fear learning, Depress.
Anxiety, 33, 907-916; doi: 10.1002/da.22497.
51.Gerenu, G., Martisova, E., Ferrero, H.,
Carracedo, M., Rantamaki, T., Ramirez, M. J., and Gil-Bea, F. J. (2017)
Modulation of BDNF cleavage by plasminogen-activator inhibitor-1
contributes to Alzheimer’s neuropathology and cognitive deficits,
Biochim. Biophys. Acta Mol. Basis Dis., 1863, 991-1001;
doi: 10.1016/j.bbadis.2017.01.023.
52.Sasi, M., Vignoli, B., Canossa, M., and Blum, R.
(2017) Neurobiology of local and intercellular BDNF signaling,
Pflugers Arch., 469, 593-610; doi:
10.1007/s00424-017-1964-4.
53.Menshanov, P. N., Lanshakov, D. A., and Dygalo,
N. N. (2015) ProBDNF is a major product of bdnf gene expressed
in the perinatal rat cortex, Physiol. Res., 64,
925-934.
54.Patz, S., and Wahle, P. (2004) Neurotrophins
induce short-term and long-term changes of cortical neurotrophin
expression, Eur. J. Neurosci., 20, 701-708; doi:
10.1111/j.1460-9568.2004.03519.x.
55.Foltran, R. B., and Diaz, S. L. (2016) BDNF
isoforms: a round trip ticket between neurogenesis and serotonin? J.
Neurochem., 138, 204-221; doi: 10.1111/jnc.13658.
56.Kowianski, P., Lietzau, G., Czuba, E., Waskow,
M., Steliga, A., and Morys, J. (2018) BDNF: a key factor with
multipotent impact on brain signaling and synaptic plasticity, Cell.
Mol. Neurobiol., 38, 579-593; doi:
10.1007/s10571-017-0510-4.
57.Mizui, T., Ishikawa, Y., Kumanogoh, H., and
Kojima, M. (2016) Neurobiological actions by three distinct subtypes of
brain-derived neurotrophic factor: multi-ligand model of growth factor
signaling, Pharmacol. Res., 105, 93-98; doi:
10.1016/j.phrs.2015.12.019.
58.Garcia, K. L., Yu, G., Nicolini, C., Michalski,
B., Garzon, D. J., Chiu, V. S., Tongiorgi, E., Szatmari, P., and
Fahnestock, M. (2012) Altered balance of proteolytic isoforms of
pro-brain-derived neurotrophic factor in autism, J. Neuropathol.
Exp. Neurol., 71, 289-297; doi:
10.1097/NEN.0b013e31824b27e4.
59.Mizui, T., Hattori, K., Ishiwata, S., Hidese, S.,
Yoshida, S., Kunugi, H., and Kojima, M. (2019) Cerebrospinal fluid BDNF
pro-peptide levels in major depressive disorder and schizophrenia,
J. Psychiatr. Res., 113, 190-198; doi:
10.1016/j.jpsychires.2019.03.024.
60.Sahay, A. S., Jadhav, A. T., Sundrani, D. P.,
Wagh, G. N., and Joshi, S. R. (2019) Differential expression of nerve
growth factor (NGF) and brain derived neurotrophic factor (BDNF) in
different regions of normal and preeclampsia placentae, Clin. Exp.
Hypertens., 14, 1-5; doi: 10.1080/10641963.2019.1665677.
61.Xiong, J., Zhou, L., Yang, M., Lim, Y., Zhu, Y.
H., Fu, D. L., Li, Z. W., Zhong, J. H., Xiao, Z. C., and Zhou, X. F.
(2013) ProBDNF and its receptors are upregulated in glioma and inhibit
the growth of glioma cells in vitro, Neuro. Oncol.,
15, 990-1007; doi: 10.1093/neuonc/not039.
62.Barcelona, P. F., and Saragovi, H. U. (2015) A
pro-nerve growth factor (proNGF) and NGF binding protein,
alpha2-macroglobulin, differentially regulates p75 and TrkA receptors
and is relevant to neurodegeneration ex vivo and in vivo,
Mol. Cell. Biol., 35, 3396-3408; doi:
10.1128/MCB.00544-15.
63.Budni, J., Bellettini-Santos, T., Mina, F.,
Garcez, M. L., and Zugno, A. I. (2015) The involvement of BDNF, NGF and
GDNF in aging and Alzheimer’s disease, Aging Dis.,
6, 331-341; doi: 10.14336/AD.2015.0825.
64.Lebrun-Julien, F., Bertrand, M. J., De Backer,
O., Stellwagen, D., Morales, C. R., Di Polo, A., and Barker, P. A.
(2010) ProNGF induces TNFalpha-dependent death of retinal ganglion
cells through a p75NTR non-cell-autonomous signaling pathway, Proc.
Natl. Acad. Sci. USA, 107, 3817-3822; doi:
10.1073/pnas.0909276107.
65.Fahnestock, M., Michalski, B., Xu, B., and
Coughlin, M. D. (2001) The precursor pro-nerve growth factor is the
predominant form of nerve growth factor in brain and is increased in
Alzheimer’s disease, Mol. Cell. Neurosci., 18,
210-220; doi: 10.1006/mcne.2001.1016.
66.Sahay, A. S., Sundrani, D. P., Wagh, G. N.,
Mehendale, S. S., and Joshi, S. R. (2015) Neurotrophin levels in
different regions of the placenta and their association with birth
outcome and blood pressure, Placenta, 36, 938-943; doi:
10.1016/j.placenta.2015.06.006.
67.Kim, K. C., Friso, S., and Choi, S. W. (2009) DNA
methylation, an epigenetic mechanism connecting folate to healthy
embryonic development and aging, J. Nutr. Biochem., 20,
917-926; doi: 10.1016/j.jnutbio.2009.06.008.
68.Kulkarni, A., Dangat, K., Kale, A., Sable, P.,
Chavan-Gautam, P., and Joshi, S. (2011) Effects of altered maternal
folic acid, vitamin B12 and docosahexaenoic acid on placental global
DNA methylation patterns in Wistar rats, PLoS One, 6,
e17706; doi: 10.1371/journal.pone.0017706.