Variations in the Expression of Terminal Oligosaccharide Units and Glycosylation of Poly(N-acetyllactosamine) Chain in the Helicobacter pylori Lipopolysaccharide upon Colonization of Rhesus Macaques
A. V. Perepelov1,a*, S. N. Senchenkova1, and Y. A. Knirel1
1Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 119991 Moscow, Russia* To whom correspondence should be addressed.
Received October 4, 2019; Revised November 29, 2019; Accepted November 30, 2019
Helicobacter pylori is an important human pathogen that causes gastritis, gastric and duodenal ulcers, and gastric cancer. O-polysaccharides of H. pylori lipopolysaccharide (LPS) are composed of (β1→3)-poly(N-acetyllactosamine) (polyLacNAc) decorated with multiple α-L-fucose residues. In many strains, their terminal LacNAc units are mono- or di-fucosylated to mimic Lewis X (Lex) and/or Lewis Y (Ley) oligosaccharides. The studies in rhesus macaques as a model of human infection by H. pylori showed that this bacterium adapts to the host during colonization by expressing host Lewis antigens. Here, we characterized LPS from H. pylori strains used in the previous study, including the parental J166 strain and the three derivatives (98-149, 98-169, and 98-181) isolated from rhesus macaques after long-term colonization. Chemical and NMR spectroscopic analyses of the LPS showed that the parent strain expressed Lex, Ley, and H type 1 terminal oligosaccharide units. The daughter strains were similar to the parental one in the presence of the same LPS core and fucosylated polyLacNAc chain of the same length but differed in the terminal oligosaccharide units. These were Lex in the isolates 98-149 and 98-169, which corresponded to the Lea phenotype of the host animals, and Ley was found in the 98-181 isolate from the macaque characterized by the Leb phenotype. As Lea and Leb are isomers of Lex and Ley, respectively, the observed correlation confirmed adaptation of the expression of terminal oligosaccharide units in H. pylori strains to the properties of the host gastric mucosa. The 98-181 strain also acquired glucosylation of the polyLacNAc chain and was distinguished by a lower expression of fucosylated internal LacNAc units (internal Lex) as a result of decoration of polyLacNAc with β-glucopyranose, which may also play a role in the bacterial adaptation.
KEY WORDS: Helicobacter pylori, lipopolysaccharide, O-polysaccharide, poly(N-acetyllactosamine), glucosylation, Lewis antigen expression, bacterial adaptation, rhesus macaqueDOI: 10.1134/S0006297920020108
Abbreviations: COSY, correlation spectroscopy; dd-Hep and ld-Hep, d-glycero- and l-glycero-d-manno-heptose; ESI MS, electrospray ionization mass spectrometry; GLC, gas-liquid chromatography; Kdo, 3-deoxy-d-manno-oct-2-ulosonic acid; LacNAc, N-acetyllactosamine; Lea, Leb, Lex, Ley, and H-1, Lewis a, Lewis b, Lewis X, Lewis Y and H type 1 antigens, respectively; LPS, lipopolysaccharide; PEtn, 2-aminoethyl phosphate; polyLacNAc, (β1→3)-poly(N-acetyllactosamine).
Helicobacter pylori is an important pathogenic microorganism [1], which causes chronic gastritis in humans.
Infection with bacterium is associated with a broad spectrum of
clinical outcomes [1, 2],
including development of peptic ulcers and increased risk of gastric
cancer. Like other Gram-negative bacteria, H. pylori contains
lipopolysaccharide (LPS) on its outer membrane, which is the major
target of the host immune system. LPS usually consists of three
distinct regions: lipid A, core oligosaccharide, and
O-polysaccharide.
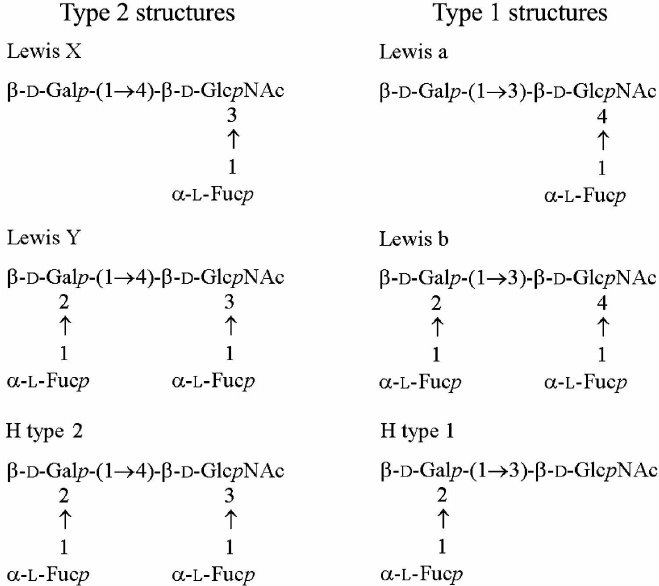
The structures of the lipid A moiety [3] and core oligosaccharide [4] of H. pylori LPS have been established. A low degree of phosphorylation and an unusual acylation pattern in lipid A contributes to the low endotoxic activity and low immune response observed with H. pylori LPS [5]. Generally, O-polysaccharides of H. pylori strains are built of (β1→3)-poly(N-acetyllactosamine) (polyLacNAc) decorated with multiple α-L-fucose residues or, in some strains, with additional glucose or galactose residues [6-11]. The terminal units of H. pylori O-polysaccharides are formed by mono- or di-fucosylated N-acetyllactosamine (LacNAc) and mimic Lewis X (Lex) and/or Lewis Y (Ley) antigens (Fig. 1) [6-12]. Some other H. pylori strains contain Lewis a (Lea), Lewis b (Leb), and H type I (H-1) antigens (Fig. 1) [13].
Fig. 1. Structures of type 2 and type 1 oligosaccharides. Lewis X, Lewis Y, and H type 1 antigens were found in the LPS of studied H. pylori strains.
Helicobacter pylori cells are able to switch their Lewis antigen phenotype during long-term colonization of rhesus macaques (Macaca mulatta) in order to adapt to their host, thus providing the evidence for the selection of bacterial phenotypes [14]. However, no structural studies have been performed to clarify the molecular basis of this adaptation. Here, we present the results of chemical, NMR spectroscopic, and electrospray ionization (ESI) mass spectrometric analyses of the O-polysaccharides, including internal and terminal oligosaccharide units, and core oligosaccharides in H. pylori strains isolated after long-term colonization of rhesus macaques as compared to the parental strain used for the infection. These data confirm the evolution of Lewis antigen expression by H. pylori strains in rhesus macaques.
MATERIALS AND METHODS
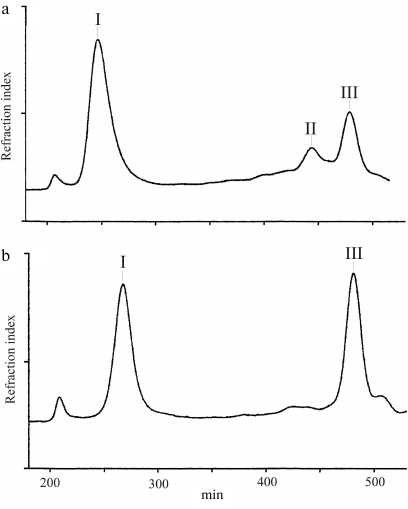
Bacterial strains and their cultivation; LPS isolation and degradation. Inoculating strain J166 and three isolates (98-149 and 98-169 from macaques expressing Lea and 98-181 from a macaque expressing Leb) recovered 40 weeks post-colonization were used in the experiments. The strains were grown on blood agar to produce bacterial biomass as described previously [15]. LPS samples were isolated by extraction with hot aqueous phenol [16] and degraded with 0.1 M sodium acetate buffer, pH 4.2, for 2 h at 100°C. The water-soluble carbohydrate portion was fractionated by gel-permeation chromatography on a Sephadex G-50 Superfine column (70 × 2.6 cm, void volume 200 ml) in 0.05 M pyridinium acetate, pH 4.5, at a flow rate of 0.5 ml/min (Fig. 2). Elution was monitored with a Waters (USA) differential refractometer, and 10-ml fractions were collected. The products obtained from each LPS sample were polysaccharides (fraction I) and truncated core oligosaccharide (fraction III); additional core oligosaccharides (fraction II) were isolated from the LPS of strains J166 and 98-169.
Fig. 2. Sephadex G-50 elution profiles of the LPS carbohydrate products from H. pylori strains 98-149 (a) and 98-169 (b). Fraction I is polyLacNAc-type O-polysaccharide attached to the core; fractions II and III are core oligosaccharides (see Fig. 3 for the corresponding structures).
Sugar and methylation analyses. Hydrolysis was performed with 2 M trifluoroacetic acid (120°C, 2 h), and resulting monosaccharides were identified by gas-liquid chromatography (GLC) as alditol acetate derivatives using a Hewlett-Packard 5880 instrument (USA) on a DB-5 fused-silica capillary column (25 m × 0.25 mm) using a temperature gradient of 160°C (1 min) to 250°C at 3°C/min.
Methylation was performed using methyl iodide in dimethyl sulfoxide in the presence of sodium methylsulfinylmethanide (CH3SOCH2Na) [17]. Hydrolysis was performed as in sugar analysis; partially methylated monosaccharides were reduced with NaBD4, converted to alditol acetates, and analyzed by GLC-MS on a Hewlett Packard 5890 chromatograph (USA) equipped with a NERMAG R10-10L mass spectrometer (France) using the above DB-5 column and a temperature gradient of 130°C (1 min) to 250°C at 3°C/min.
Smith degradation. O-polysaccharide (2 mg) from the 98-181 strain was oxidized with 0.1 M NaIO4 (0.3 ml) at 20°C for 48 h in the dark; the excess of the oxidant was removed by adding ethylene glycol (0.05 ml). The product was reduced with NaBH4 (6 mg) at 20°C for 2 h, desalted by gel-permeation chromatography on a Fractogel TSK HW-40S column (24 × 1 cm) in water, and hydrolyzed with 2% acetic acid (2 h, 100°C); the resulting modified polysaccharide was isolated using the same Fractogel column.
NMR spectroscopy and mass spectrometry. NMR spectra were recorded in 99.96% D2O at 60°C using a JEOL EX-270 instrument. Prior to the measurements, the samples were deuterium-exchanged by freeze-drying twice from 99.9% D2O. Chemical shifts are reported in ppm relative to the internal standard sodium 3-trimethylsilylpropanoate-2,2,3,3-d4 (δH 0.00).
Electrospray ionization mass spectrometry (ESI MS) was performed in the negative ion mode using a VG Quattro triple quadrupole mass spectrometer (Micromass, UK) with acetonitrile as a mobile phase at a flow rate of 10 μl/min. The samples were dissolved in 50% aqueous acetonitrile at a concentration of ~50 pmol/μl, and 10 μl was injected via a syringe pump into the electrospray source.
RESULTS AND DISCUSSION
LPS degradation and characterization of core oligosaccharides. Mild acidic degradation of LPS samples from H. pylori J166 and three daughter strains (98-149, 98-169, and 98-181) followed by gel-permeation chromatography on Sephadex G-50 (Fig. 2) yielded polyLacNAc-type O-polysaccharide (fraction I) and one or two core oligosaccharide fractions (fraction III from strains 98-149 and 98-181 and fractions II and III from strains J166 and 98-169). All products were studied by sugar analysis; the oligosaccharides were analyzed by ESI MS; O-polysaccharides were studied by methylation analysis (table) and NMR spectroscopy.
Methylation analysis of fraction I of O-polysaccharides. GLC retention
times for partially methylated alditol acetates are referred to
2,3,4-tri-O-methylfucose (2,3,4-Me3-Fuc, 1.00) and
glucitol hexaacetate (2.67)
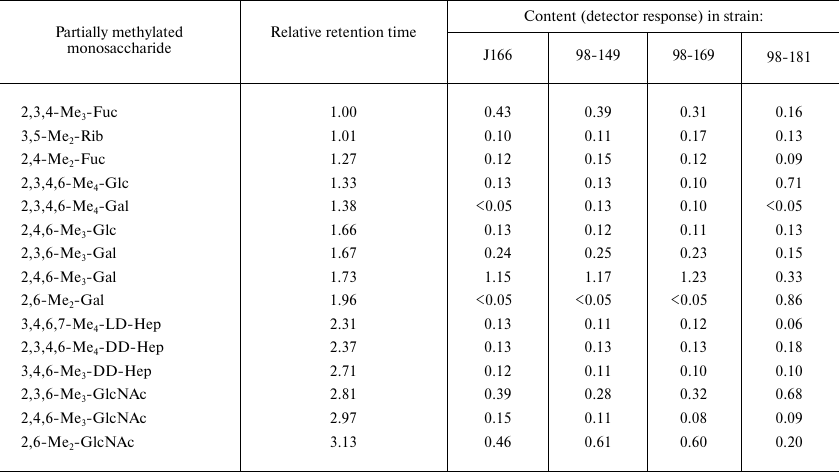
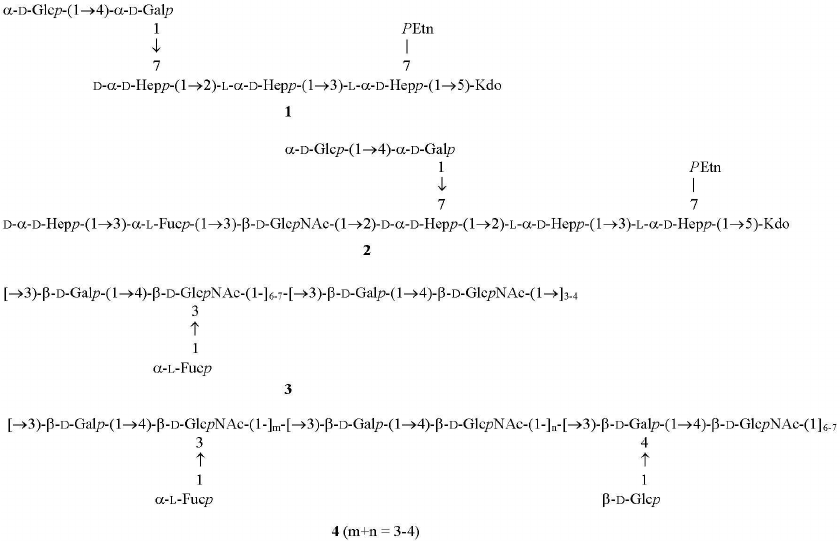
Sugar analysis indicated that the major constituents of oligosaccharides in fractions III from each strain were glucose, galactose, d-glycero-d-manno-heptose (dd-Hep), and l-glycero-d-manno-heptose (ld-Hep). Their ESI mass spectra showed the presence of a main compound with the molecular mass of 1242.4 Da. Taking into account that data of sugar and ESI MS analyses, it was concluded that this compound was truncated core oligosaccharide GlcGal(dd-Hep)(ld-Hep)2KdoPEtn, where Kdo is 3-deoxy-d-manno-oct-2-ulosonic acid in the anhydro form, and PEtn is 2-aminoethyl phosphate. A compound of the same composition has been derived earlier from LPSs of other H. pylori strains, and its structure 1 has been established (Fig. 3) [7, 13].
Fig. 3. Structures of fraction III truncated core oligosaccharide from all studied H. pylori strains (1), major fraction II core oligosaccharide from H. pylori strains J166 and 98-169 (2), and fraction I O-polysaccharides from H. pylori strains J166, 98-149, 98-169 (3), and 98-181 (4). In oligosaccharides 1 and 2, Kdo is present in the anhydro form. Distribution of glycosylated and non-glycosylated LacNAc units in polysaccharides 3 and 4 was not determined.
Major fraction II oligosaccharide obtained from the LPSs of H. pylori strains J166 and 98-169 differed from that of fraction III by the presence of fucose and GlcNAc and a higher content of dd-Hep. Its sugar composition and molecular mass (1946.6 Da) determined by ESI MS corresponded to the core oligosaccharide from H. pylori strains 26695 and Sydney (structure 2 in Fig. 3) [4, 18]. Fraction II also contained compounds with one or several additional hexose and ribose residues that had similar composition to H. pylori core oligosaccharides reported earlier [4].
Sugar and methylation analyses of fraction I O-polysaccharides from each strain (table) showed the presence of all core constituents, including terminal glucose, 4-substituted galactose, 2-substituted ld-Hep, 7-substituted dd-Hep (site of core elongation), 2,7-disubstituted dd-Hep, 3-substituted GlcNAc, and 3-substituted fucose. These components were minor, so we suggested that fraction I represented O-polysaccharide attached to the LPS core.
Characterization of O-polysaccharides. Sugar and methylation analyses of fraction I polysaccharide from the LPSs of H. pylori strains J166, 98-149, and 98-169 (table) revealed typical (1→3)-polyLacNAc-based structure 3 with some LacNAc units fucosylated at position 3 of GlcNAc (Fig. 3) (compare to the published data [7, 18-20]). As judged from the ratio between 4-substituted and 3,4-disubstituted GlcNAc residues (table), the degree of fucosylation was 54, 69, and 65% in the J166, 98-149, and 98-169 strains, respectively, and hence, fucosylated LacNAc units were predominant. The ratio between methylated derivatives of 3-substituted Gal of LacNAc units and heptose residues in the core indicated the presence, on the average, of 10 to 11 interior LacNAc units in each polysaccharide. The 1H NMR spectra of the O-polysaccharides showed major signals for anomeric protons of β-GlpcNAc (δ 4.74, J1,2 8.5 Hz), β-Galp (δ 4.49, J1,2 8 Hz), and α-Fucp (δ 5.04, J1,2 3 Hz) (compare to the published data [7, 18-20]). These results confirmed the poly(α-fucosylated) polyLacNAc structure 3 (Fig. 3).
Whereas no significant structural difference was observed between the fraction I polysaccharides from strains J166, 98-149 and 98-169, the O- polysaccharide from H. pylori 98-181 differed in a significantly higher content of glucose (82% vs. 22-29% in the three other strains). Its methylation analysis showed the presence of terminal glucose and 3,4-disubstituted galactose as the major components, whereas 3-substituted galactose, which dominated in the O-polysaccharides with structure 3, became a minor component (table). In addition to the signals from the anomeric protons observed in the O-polysaccharides from the strains J166, 98-149, and 98-169 (see above), the 1H NMR spectra of the polysaccharide from the strain 98-181 showed a major signal for H1 of β-Glcp (δ 4.88, J1,2 7.5 Hz).
Taken together, these data indicated that the O-polysaccharide from H. pylori strain 98-181 had structure 4, which is distinguished by the decoration of LacNAc units with β-glucose attached at position 4 of the galactose residue (Fig. 3). As judged from the ratio of 3-substituted to 3,4-disubstituted galactose residues (table), the degree of glucosylation was ~70%. The length of the polyLacNAc chain in the strain 98-181 was similar to that in the three other studied strains, whereas the degree of fucosylation was lower (~30%).
Structure 4 was confirmed by Smith degradation of the O-polysaccharide, which resulted in the destruction and subsequent removal of terminal fucose and glucose residues, yielding linear polyLacNAc identified by methylation and 1H NMR spectroscopy (data not shown). Glucosylation of polyLacNAc at position 4 of β-Galp is a new feature of H. pylori LPS, whereas glucosylation or galactosylation of β-GlcpNAc at position 6 has been reported before [9].
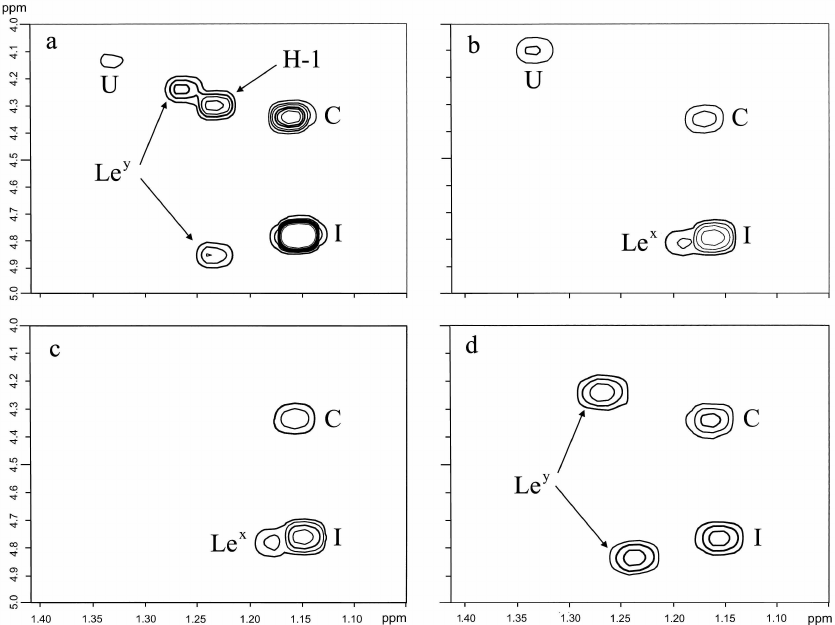
Characterization of terminal oligosaccharide units and correlation with the host phenotype. The structures of the terminal non-reducing units of the O-polysaccharides were studied using two-dimensional 1H,1H correlation spectroscopy (COSY) as described by us earlier [7, 18]. This approach based on the analysis of the characteristic patterns of Fuc H5/H6 correlations was found to be useful for the identification of terminal oligosaccharide units in the O-polysaccharides of H. pylori.
Fucose residues at conserved positions in the core region (C) (H5/H6 at δ 4.33/1.14) and interior fucosylated LacNAc unit (I) (H5/H6 at δ 4.80/1.13) were identified in O-polysaccharides of all strains studied in this work (Fig. 4). In the parental strain J166, additional fucose residues were present to form terminal Ley (H5/H6 at δ 4.87/1.22 and 4.24/1.25) and H-1 (H5/H6 at δ 4.29/1.24) (Fig. 4a). None of the daughter strains showed the same Fuc H5/H6 correlation pattern as the parent strain, being devoid of one or two terminal oligosaccharide units. Thus, H-1 was lost from all the three strains (Fig. 4, b-d), and only the strain 98-181 retained Ley (Fig. 4d). The strains 98-149 and 98-169 expressed Lex in place of Ley (Fig. 4, b and c).
Fig. 4. Fragment of the 1H,1H COSY spectra of the O-polysaccharides from H. pylori strains J166 (a), 98-149 (b), 98-169 (c), and 98-181 (d). The spectra show H5/H6 correlations of fucose residues in the core (C), interior units of the O-polysaccharides (I), terminal Lewis X (Lex), Lewis Y (Ley) and H type 1 (H-1), and at an unknown position (U).
Expression of Ley by the isolate 98-181 does not contradict the secretion status of the host macaque characterized by the Leb phenotype. The lack of expression of the terminal Lex by the 98-181 strain was accompanied by a lower level of expression of fucosylated internal LacNAc units, which can be considered as internal Lex. Accordingly, the isolates 98-149 and 98-169 were characterized by higher expression of both terminal and internal Lex units, which corresponded to the Lea phenotype of the host animals. Therefore, because Lea and Leb are isomers of Lex and Ley (Fig. 1), respectively we suggested that the observed correlations were adaptations (or selection) of H. pylori strains to the gastric mucosa of a particular host.
Acknowledgements.The authors thank Prof. P.-E. Jansson (Karolinska Institute, Clinical Research Center, Huddinge University Hospital, Huddinge, Sweden) for the access to laboratory equipment, including an NMR spectrometer and a GLC-mass spectrometer, M. J. Blaser (New York University, New York, USA) for providing H. pylori strains, and A. P. Moran (National University of Ireland, Galway, Ireland) for cultivation of bacteria and valuable discussion.
Conflict of interest. The authors declare no conflict of interest in financial or any other area.
Ethical approval. This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
1.Kusters, J. G., van Vliet, A. H., and Kuipers, E.
J. (2006) Pathogenesis of Helicobacter pylori infection,
Clin. Microbiol. Rev., 19, 449-490.
2.Chmiela, M., and Kupcinskas, J. (2019) Pathogenesis
of Helicobacter pylori infection, Helicobacter, 24
(Suppl. 1), e12638.
3.Moran, A. P., Lindner, B., and Walsh, E. J. (1997)
Structural characterization of the lipid A component of Helicobacter
pylori rough- and smooth-form lipopolysaccharides, J.
Bacteriol., 179, 6453-6463.
4.Altman, E., Chandan, V., Li, J., and Vinogradov, E.
(2011) A reinvestigation of the lipopolysaccharide structure of
Helicobacter pylori strain Sydney (SS1), FEBS J.,
278, 3484-3493.
5.Moran, A. P. (1996) The role of lipopolysaccharide
in Helicobacter pylori pathogenesis, Aliment. Pharmacol.
Ther., 10 (Suppl. 1), 39-50.
6.Moran, A. P., and Aspinall, G. O. (1998) Unique
structural and biological features of Helicobacter pylori
lipopolysaccharides, Prog. Clin. Biol. Res., 397,
37-49.
7.Knirel, Y. A., Kocharova, N. A., Hynes, S. O.,
Widmalm, G., Andersen, L. P., Jansson, P.-E., and Moran, A. P. (1999)
Structural studies on lipopolysaccharides of serologically non-typable
strains of Helicobacter pylori, AF1 and 007, expressing Lewis
antigenic determinants, Eur. J. Biochem., 266,
123-131.
8.Wang, G., Ge, Z. M., Rasko, D. A., and Taylor, D.
E. (2000) Lewis antigens in Helicobacter pylori: biosynthesis
and phase variation, Mol. Microbiol., 36,
1187-1196.
9.Monteiro, M. A. (2001) Helicobacter pylori:
a wolf in sheep’s clothing: the glycotype families of
Helicobacter pylori lipopolysaccharides expressing histo-blood
groups: structure, biosynthesis, and role in pathogenesis, Adv.
Carbohydr. Chem. Biochem., 57, 99-158.
10.Moran, A. P. (2008) Relevance of fucosylation and
Lewis antigen expression in the bacterial gastroduodenal pathogen
Helicobacter pylori, Carbohydr. Res., 343,
1952-1965.
11.Chmiela, M., Miszczyk, E., and Rudnicka, K.
(2014) Structural modifications of Helicobacter pylori
lipopolysaccharide: an idea for how to live in peace, World J.
Gastroenterol., 20, 9882-9897.
12.Li, H., Liao, T., Debowski, A. W., Tang, H.,
Nilsson, H. O., Stubbs, K. A., Marshall, B. J., and Benghezal, M.
(2016) Lipopolysaccharide structure and biosynthesis in Helicobacter
pylori, Helicobacter, 21, 445-461.
13.Monteiro, M. A., Chan, K. H. N., Rasko, D. A.,
Taylor, D. E., Zheng, P. Y., Appelmelk, B. J., Wirth, H. P., Yang, M.
Q., Blaser, M. J., Hynes, S. O., Moran, A. P., and Perry, M. B. (1998)
Simultaneous expression of type 1 and type 2 Lewis blood group antigens
by Helicobacter pylori lipopolysaccharides, J. Biol.
Chem., 273, 11533-11543.
14.Wirth, H.-P., Manqiao, Y., Edgardo, S.-V., Berg,
D. E., Dubois, A., and Blaser, M. J. (2006) Host Lewis
phenotype-dependent Helicobacter pylori Lewis antigen expression
in rhesus macaques, FASEB J., 20, 1534-1536.
15.Moran, A. P., Helander, I. M., and Kosunen, T. U.
(1992) Compositional analysis of Helicobacter pylori rough-form
lipopolysaccharides, J. Bacteriol., 174,
1370-1377.
16.Westphal, O., and Jann, K. (1965) Bacterial
lipopolysaccharides extraction with phenol–water and further
applications of the procedure, Methods Carbohydr. Chem.,
5, 83-91.
17.Hakomori, S.-I. (1964) A rapid permethylation of
glycolipid and polysaccharide catalyzed by methylsulfinyl carbanion in
dimethyl sulfoxide, J. Biochem. (Tokyo), 55, 205-208.
18.Moran, A. P., Knirel, Y. A., Senchenkova, S. N.,
Widmalm, G., Hynes, S. O., and Jansson, P.-E. (2002) Phenotypic
variation in molecular mimicry between Helicobacter pylori
lipopolysaccharides and human gastric epithelial cell surface
glycoforms. Acid-induced phase variation in Lewis(x) and Lewis(y)
expression by H. pylori lipopolysaccharides, J. Biol.
Chem., 277, 5785-5795.
19.Monteiro, M. A., Rasko, D., Taylor, D. E., and
Perry, M. B. (1998) Glucosylated N-acetyllactosamine O-antigen chain in
the lipopolysaccharide from Helicobacter pylori strain UA861,
Glycobiology, 8, 107-112.
20.Aspinall, G. O., Monteiro, M. A., Pang, H.,
Walsh, E. J., and Moran, A. P. (1996) Lipopolysaccharide of the
Helicobacter pylori type strain NCTC 11637 (ATCC 43504):
structure of the O-antigen chain and core oligosaccharide regions,
Biochemistry, 35, 2489-2497.