Ommochromes from the Compound Eyes of Insects: Physicochemical Properties and Antioxidant Activity
A. E. Dontsov1, N. L. Sakina1, M. A. Yakovleva1, A. I. Bastrakov2, I. G. Bastrakova3, A. A. Zagorinsky4, N. A. Ushakova2, T. B. Feldman1,5, and M. A. Ostrovsky1,5,a*
1Emanuel Institute of Biochemical Physics, Russian Academy of Sciences, 119334 Moscow, Russia2Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia
3All-Russian Research Institute of Silviculture and Mechanization of Forestry, 141200 Pushkino, Moscow Region, Russia
4Russian Forest Protection Center, 141202 Pushkino, Moscow Region, Russia
5Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received April 1, 2020; Revised May 5, 2020; Accepted May 8, 2020
The objective of this study was screening of ommochromes from the compound eyes of insects and comparison of their antioxidant properties. Ommochromes were isolated in preparative quantities from insects of five different families: Stratiomyidae, Sphingidae, Blaberidae, Acrididae, and Tenebrionidae. The yield of ommochromes (dry pigment weight) was 0.9-5.4% of tissue wet weight depending on the insect species. Isolated pigments were analyzed by high-performance liquid chromatography and represented a mixture of several ommochromes of the ommatin series. The isolated ommochromes displayed a pronounced fluorescence with the emission maxima at 435-450 nm and 520-535 nm; furthermore, the emission intensity increased significantly upon ommochrome oxidation with hydrogen peroxide. The ommochromes produced a stable EPR signal consisting of a singlet line with g = 2.0045-2.0048, width of 1.20-1.27 mT, and high concentration of paramagnetic centers (> 1017 spin/g dry weight). All the investigated ommochromes demonstrated high antiradical activity measured from the degree of chemiluminescence quenching in a model system containing luminol, hemoglobin, and hydrogen peroxide. The ommochromes strongly inhibited peroxidation of the photoreceptor cell outer segments induced by visible light in the presence of lipofuscin granules from the human retinal pigment epithelium, as well as suppressed iron/ascorbate-mediated lipid peroxidation. The obtained results are important for understanding the biological functions of ommochromes in invertebrates and identifying invertebrate species that could be used as efficient sources of ommochromes for pharmacological preparations to prevent and treat pathologies associated with the oxidative stress development.
KEY WORDS: ommochromes, insects, EPR spectrometry, fluorescence, antioxidant activityDOI: 10.1134/S0006297920060048
Abbreviations: EPR, electron paramagnetic resonance; HPLC, high performance liquid chromatography; TBA, thiobarbituric acid.
INTRODUCTION
Ommochromes are invertebrate pigments containing phenoxazine/phenothiazine rings. Ommochromes are classified into two major subgroups – ommatins and ommins – that represent either dimers or oligomers of kynurenine derivatives, respectively [1, 2]. Ommatins and ommins in vivo are typically dark-colored yellow-brown or purple pigments. Insects use ommochromes for the coloration of different body parts in mimicry. Thus, xanthommatin and its derivatives (such as ommatin D) are responsible for the body color and its changes in arthropods [3-5]. Significant amounts of ommochromes are present in the compound eyes of arthropods, where they perform an important function. In particular, we demonstrated earlier that the content of ommochromes in the eye of the opossum shrimp Mysis relicta reaches 80 mg dry weight per 1 ml or 200 µg dry weight per 1 mg protein [6]. Compound eyes of most arthropods consist of multiple small eyelets (ommatidia), each with its own photoreceptor cells (rhabdomeres). In addition to the light-sensitive retinal-containing visual pigments, all vision organs contain light-non-sensitive screening pigments – ommochromes. Their main function in the compound eyes is to filter and absorb light [7, 8]. Ommochromes participate in the formation of the eye spectral sensitivity by absorbing or transmitting light of particular wavelengths. Moreover, by absorbing scattered light, ommochromes increase the contrast and the sharpness of the image.
In addition to the optical function, ommochromes play a protective antioxidant role [9-12], which is necessary because some light quanta, especially those in the violet and blue regions of the spectrum, carry high energy and present a potential danger, especially for the retinal photoreceptor cells (the so-called blue light hazard). The photodamage of insect eye structures has its own features. First, unlike in vertebrates, prolonged exposure to light can result in the accumulation of phagosomes [13] containing a large amount of unsaturated peroxidation-prone fatty acids in the eye retinular cells. Second, the eye optical medium in many insects transmits UV light, which is an active exogenous prooxidant factor. In this case, illumination with UV light or intense visible light could result in the accumulation of toxic peroxidation products capable of penetrating rhabdom membranes and causing their damage. Hence, the probability of photodamage in the ommatidia of a compound eye is likely higher than in the eyes of vertebrates. Hence, additional protection of the compound eye cells from peroxidation is essential. Ommochromes can play this protective role in the eyes of arthropods. It is known that crustacean and insect species containing large amounts of screening pigments are extremely resistant to prooxidant factors [6, 10, 14]. Furthermore, as we have shown using cells of compound eye from M. relicta, the resistance to the action of prooxidant factors was not associated with a higher content of low-molecular-weight antioxidants and antioxidant enzymes, but was rather due to the presence of a large number of ommochrome-containing granules [14].
Ommochromes can be either electron donors or electron acceptors and function as efficient antiradical molecules [15-17]. One of the mechanisms underlying the protective effect of ommochromes against the photodamage of visual cells could be their reaction with singlet oxygen, which is an extremely toxic oxidant. We have demonstrated previously that ommochromes from the eye of Pandalus latirostris shrimp quenched the photosensitized luminescence of singlet oxygen [18]. At the same, one cannot rule out that ommochromes could be generators of singlet oxygen. Such possibility was reported, for example, for kynurenine – a precursor of ommochromes [19-21]. However, there are no published data on the excitation of ommochromes, triplet formation, and singlet oxygen generation. Most likely, ommochromes only act as singlet oxygen quenchers.
It is commonly accepted now that the antioxidant activity of ommochromes is due to their ability to neutralize reactive oxygen species and to bind transition metals into inactive complexes [9, 11]. We demonstrated previously that ommochromes from the black soldier fly Hermetia illucens were capable of quenching luminol luminescence induced by hydrogen peroxide in the presence of hemoglobin [12] with the quenching constant (>104 M–1) [22] that was comparable with similar constants for synthetic antioxidants, such as mexidol and hydroxy(alkoxy)-2-aminobenzothiazole derivatives [23].
Arthropods, such as crustaceans and insects, are a good source of ommochromes. These natural antioxidants can be used for practical purposes, for example, in pharmacology. In this study, we isolated ommochromes from different species of feed insects and investigated their physicochemical and antioxidant properties.
MATERIALS AND METHODS
Isolation of ommochromes from insect heads. Ommochromes were isolated from five insect species. Black soldier fly (Hermetia illucens, Stratiomyidae family) and its larvae are widely used as a livestock feed and in conversion of biological waste into food protein, fats, chitin, and melanin. These flies are maintained as a pure line at the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences. The procedure for the cultivation of flies includes the following stages: breeding adult flies at the insectarium under controlled conditions; incubating eggs and producing larvae in an incubator; growing larvae in a container with a nutrient substrate; producing prepupae, pupae, and imago. Adult flies live for five to eight days. At the end of the life cycle, dead flies were collected, frozen, and stored at –180°C.The genome of Hermetia illucens is available from the GenBank (Hermetia illucens, sample H-il 1 No. KY817115).
Mealworm beetle (Tenebrio molitor, Tenebrionidae family) is currently one of the most popular species used for feeding various exotic animals. Large-scale production facilities exist in Europe, China, and the USA. A culture of mealworm beetles was maintained under laboratory conditions at the Severtsev Institute of Ecology and Evolution.
Marble cockroach (Nauphoeta cinerea, Blaberidae family) is also commonly used for feeding insect-eating animals. A culture of marble cockroaches was maintained under laboratory conditions at the Severtsev Institute of Ecology and Evolution.
The larvae of the tobacco hornworm butterfly (Manduca sexta, Sphingidae family) are another popular food for exotic insect-eating animals. A culture of tobacco hornworm butterfly was maintained at the Entomology department of the Moscow Zoo.
Desert locust (Schistocerca gregaria, Acrididae family) is also used as a live food for insect-eating animals. A culture of desert locust was maintained at the Entomology department of the Moscow Zoo.
Ommochromes were extracted from the insect heads without preliminary homogenization. The heads of dead adult insects were manually separated and stored at –180°C. If necessary, insect heads were first incubated in neutral methanol (~10 g heads per 300 ml methanol) for 24 h in the dark at room temperature with periodical mixing. Following filtration, 500 ml of absolute methanol containing 1 vol % of hydrogen chloride (MeOH-HCl mixture) was added to the heads and incubated at 6°C in the dark for 48 h with periodical shaking. Next, the extract was filtered through a paper filter (Whatman, Grade 6). The produced cherry-colored filtrate was neutralized with 20% ammonium solution and centrifuged at 5,000g for 15 min. The supernatant was discarded, and fresh MeOH-HCl mixture was added to the precipitate to its complete dissolution. The precipitation procedure with ammonium was repeated twice. Finally, the ommochrome precipitate was dried in a desiccator in the presence of anhydrous calcium chloride.
Isolation of lipofuscin granules. Lipofuscin granules from the retinal pigment epithelium were isolated from the eyes of cadaveric donors using procedure suggested Boulton and Marshall [24]. Cadaveric human eyes were obtained within the framework of agreement with the Eye Tissue Bank, Fyodorov Eye Microsurgery Complex, Ministry of Health of the Russian Federation. Isolated lipofuscin granules were washed with 0.1 M K-phosphate buffer (pH 7.6), resuspended in the same buffer, and stored at –20°C. The concentration of the granules was evaluated with a hemocytometer.
Preparation of outer segments of bovine photoreceptors. Outer segments of photoreceptor cells were isolated from the bovine retina using the modified method suggested in [25]. The produced outer segments were suspended in 0.1 M K-phosphate buffer and stored at –20°C.
Preparation of cardiolipin liposomes. Cardiolipin liposomes were prepared by suspending cardiolipin in 0.1 M K-phosphate buffer, pH 7.4. Cardiolipin sodium salt (Sigma-Aldrich, USA) in methanol (5 mg/ml) was dried in a rotary evaporator and the formed lipid film was solubilized in buffer followed by thorough shaking on a Vortex-type mixer. The liposome suspension was stored at 2-4°C.
Analysis of ommochrome extracts. Isolated ommochromes were analyzed by high-performance liquid chromatography (HPLC) using a Knauer chromatograph (Germany) on a Diasphere 120 C18 column (4 × 250 mm; particle size, 5 µm). Solvent A was 10% aqueous acetonitrile containing 0.5% formic acid; solvent B was 100% acetonitrile containing 0.5% formic acid. The pigments were fractionated in a linear gradient (0-40%) of solution B in solution A for 60 min at a flow rate of 0.4 ml/min at 24°C. Eluted pigments were registered with a Knauer K-2501 UV/Vis detector and an RF-10A-xl fluorescence detector (Shimadzu, Japan). The ommochromes or standard compounds were dissolved in 100 µl of methanol containing 0.5% HCl. Tryptophan, kynurenine, 3-hydroxykynurenine, and xanthurenic acid (Sigma-Aldrich, USA) were used as standards. Xanthommatin was synthesized by autooxidation of 3-hydroxykynurenine as described in [26]. Absorption spectra were recorded with a Shimadzu UV–1601PC spectrophotometer. Fluorescence spectra were recorded with a Shimadzu RF-5301PC fluorimeter. The obtained data were processed with the RFPC version 2.0 software (Shimadzu).
Measuring the concentration of free radical centers. The parameters of the paramagnetic centers in the ommochromes were determined by the electron paramagnetic resonance (EPR) spectroscopy. Dry ommochrome samples or frozen ommochrome suspensions in K-phosphate buffer prepared using an attachment made from a piece of polyethylene tube with a length of 10-15 mm and inner diameter of 0.45 mm were used in the experiments. Each sample (0.3 ml) was quickly frozen in liquid nitrogen and stored until the measurements. To record the EPR spectra, the frozen samples were pushed out of the tubes with a plunger. The EPR spectra were recorded at 77°K with a Bruker EMX EPR spectrometer in a cylindrical resonator. The conditions for the EPR spectrum recording were: ΔH scan, – 50 Gs; H center, 3440 Gs; modulation amplitude, 3 Gs; microwave power, 20 µW. The UDA No. 5 spin concentration standard (calibration certificate No. 905/910-2012) was used for determining spin concentration.
Oxidation of ommochromes with hydrogen peroxide. The oxidative destruction of ommochromes was induced with 1.0-1.5% hydrogen peroxide. The pigment suspension (2-3 mg/ml) in 0.1 M K-phosphate buffer, pH 7.4, or ommochrome solution in MeOH-HCl (0.5-1.0 mg/ml) were incubated in the presence of hydrogen peroxide for at least 2 h. Next, the physicochemical characteristics of the original and oxidized samples were compared.
The antioxidant activity of ommochromes was determined using a homogenous hydrophilic chemiluminescence system consisting of hemoglobin, hydrogen peroxide, and luminol [27]. The latent period before the development of maximal luminescence intensity was measured. The chemiluminescence kinetics was recorded with a Shimadzu RF 5301PC spectrofluorimeter at the emission wavelength of 470 nm at room temperature. The ability of ommochromes to interact with the radicals in the aqueous phase of the model system was evaluated from chemiluminescence quenching in the latent period duration versus pigment concentration coordinates. The incubation medium contained 0.05 M K-phosphate buffer, pH 7.4, 2.0 µM hemoglobin, 100 µM luminol, 100 µM EDTA, and varying concentrations of ommochromes in 0.1 M K-phosphate buffer, pH 7.4, or in methanol-HCl solution. The reaction was initiated by adding 100 µM hydrogen peroxide. The buffer solution without ommochromes was used as a control.
The kinetics of lipid peroxidation in cardiolipin liposomes or outer segments of photoreceptor cells was determined from the accumulation of products of reaction with thiobarbituric acid (TBA-reactive substances) [28]. Lipid peroxidation was initiated either with divalent iron ions or visible light in the presence of lipofuscin granules. A KGM-24-150 halogen lamp equipped with a focusing system and a heat-absorbing filter was used for illumination within the full visible spectrum. The illumination energy was 80 mW/cm2; the illumination spectral range was 390-700 nm. Typically, the samples were illuminated at room temperature with constant mixing. The average rate of the accumulation of TBA-reactive substances was calculated by measuring the concentration of the products formed 10, 25, and 40 min after the reaction initiation.
Statistical analysis was performed using the Student’s t-test (p < 0.05). The data are presented as mean ± standard deviation.
RESULTS AND DISCUSSION
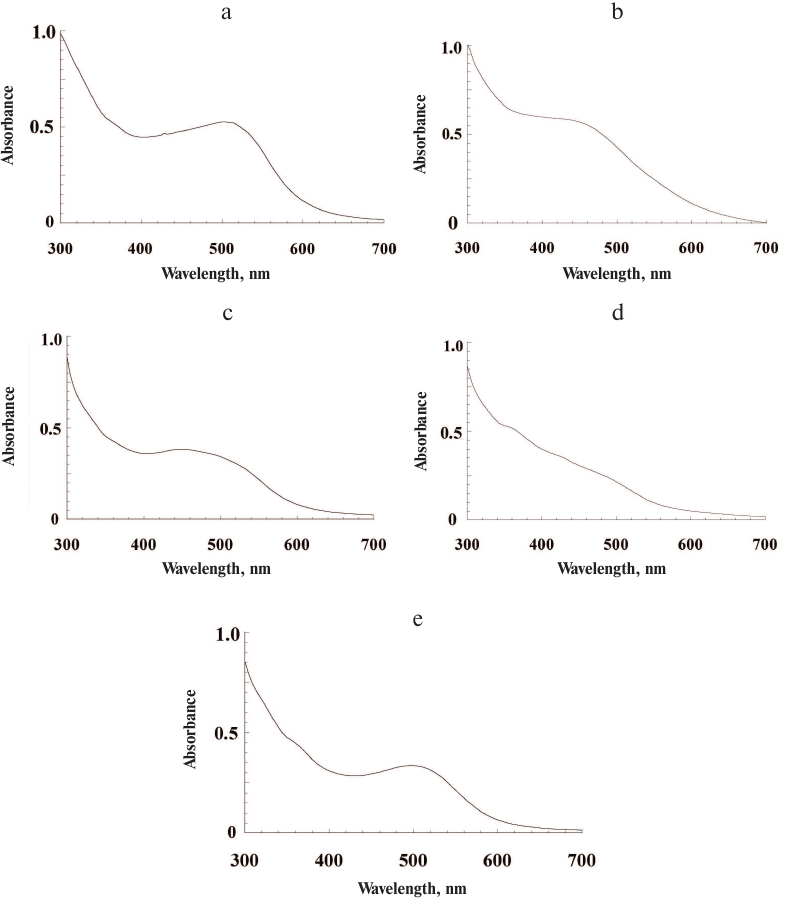
All investigated insect species contained high concentrations of ommochromes, which were extracted from the insect heads with relatively high yields. The yield of the ommochromes was 0.9, 1.4, 1.7, 4.2, and 4.8% of dry pigment weigh per initial biomass weight for the marble cockroach, desert locust, tobacco hornworm butterfly, black soldier fly, and mealworm beetle, respectively. The maxima of the absorption spectra of ommochromes in the visible range of the spectrum were between 430 and 502 nm for all the investigated insects (Fig. 1).
Fig. 1. Absorption spectra of insect ommochromes in methanol-HCL solution: a) tobacco hornworm butterfly; b) black soldier fly; c) mealworm beetle; d) desert locust; e) marble cockroach. Ommochrome concentration, 0.4-0.6 mg/ml.
The longest wavelength absorption maxima were characteristic for ommochromes from the tobacco hornworm butterfly (502 nm, Fig. 1a) and marble cockroach (497 nm, Fig. 1e). The ommochromes from the mealworm beetle displayed pronounced absorption maximum at 454 nm (Fig. 1c), while less pronounced absorption maximum at the shortest wavelength (430 nm) was observed for ommochromes from the black soldier fly (Fig. 1b). The ommochromes from the desert locust did not have any pronounced absorption maximum in the visible range and displayed only a “shoulder” around 490 nm (Fig. 1d). The absorption spectra were the same for the preparations isolated independently form the same source (3-4 independent isolations), which was likely due to the fact that the insect cultivation conditions have not been changed in the course of the experiment. The absorption maxima of ommochromes at 430-490 nm are typical for ommatins, and the absorption maxima at 520 nm and above are characteristic for ommins [1]. The absorption spectra of ommochromes from the black soldier fly, mealworm beetle, and desert locust were closer to the ommatin spectra; the absorption spectra of ommochromes from the marble cockroach and tobacco hornworm butterfly were of an intermediate type, which suggests the presence of two types of ommochromes – ommatins and ommins – in their composition.
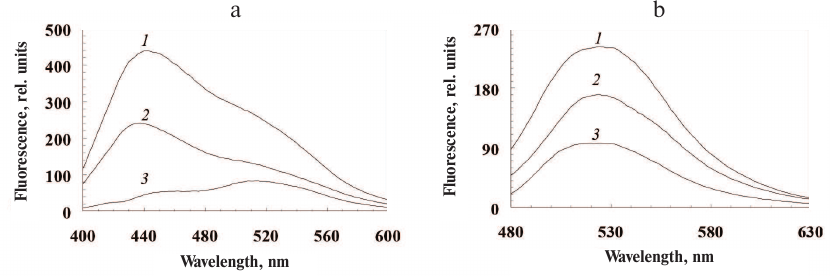
All isolated ommochromes strongly fluoresce in the visible spectral range with the two major fluorescence peaks: the short-wavelength peak with the emission maximum at 440 nm and the long-wavelength peak with the emission maximum at 530 nm (Fig. 2, a and b, respectively).
Fig. 2. Fluorescence spectra of ommochromes from the desert locust (1), tobacco hornworm butterfly (2), and marble cockroach (3) in methanol-HCl. Excitation wavelength, 380 nm (a) and 460 nm (b).
The main excitation maxima were 290 nm, 330 nm, and 380 nm for the short-wavelength fluorescence and 330 nm and 460 nm for the long-wavelength fluorescence.
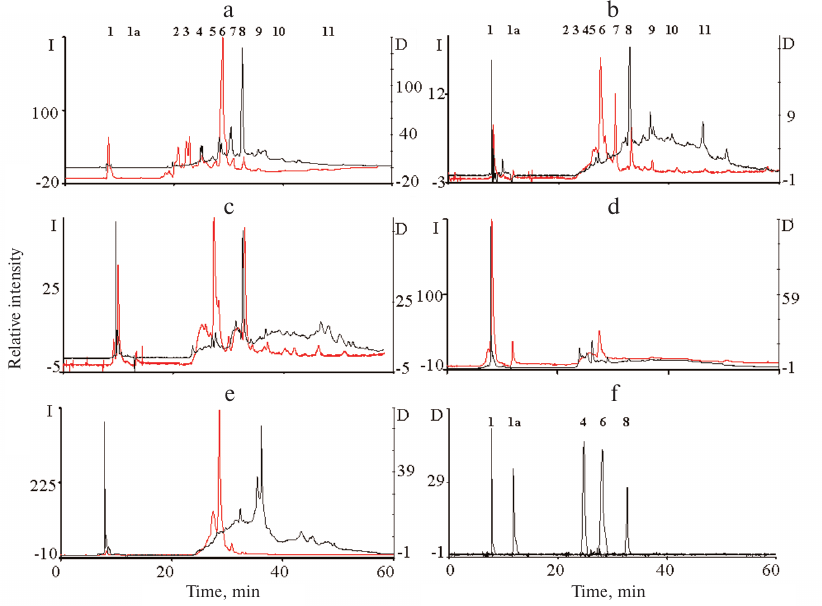
The qualitative composition of the ommochromes was examined by HPLC. The chromatograms of the ommochromes recorded by measuring absorbance at 490 nm and fluorescence (excitation wavelength, 460 nm; emission, 520 nm) are shown in Fig. 3.
Fig. 3. HPLC analysis of ommochromes extracted from the eyes of tobacco hornworm butterfly (a), black soldier fly (b), mealworm beetle (c), desert locust (d), and marble cockroach (e); (f) standard compounds. Black line, absorbance at 490 nm; y-axis, D; red line, fluorescence at 520 nm (excitation wavelength, 460 nm); y-axis, I. Peaks: 1-3) hydroxykynurenine; 1a) kynurenine; 4) tryptophan; 5) supposedly dihydroxanthommatin [29]; 6) xanthurenic acid; 7) supposedly decarboxylated xanthommatin [29]; 8) xanthommatin.
The composition of ommochromes was different in the studied insect species (Fig. 3). The ommochromes were a mixture of several compounds, mainly xanthommatin and several its derivatives. The ommochromes from the marble cockroach differ significantly in the composition from the ommochromes from the rest of insects (Fig. 3e). The ommochromes from the tobacco hornworm butterfly and black soldier fly were close in composition, as indicated both by their absorption and fluorescence (Fig. 3, a and b). However, unlike the ommochromes from the black soldier fly, the ommochromes from the tobacco hornworm butterfly included ommatin D with the absorption maximum at 490 nm, which is in agreement with the results of the study [30], in which a mixture of ommatin D and xanthommatin was identified as a chromophore in the ommochrome-binding protein in the tobacco hornworm butterfly hemolymph. The composition of ommochromes from the mealworm beetle was closer to the composition of ommochromes from the tobacco hornworm butterfly and black soldier fly than to the composition of ommochromes from the marble cockroach (Fig. 3c). It is important to note that ommochromes from the majority of investigated insects had the absorption maximum close to the absorption maximum of ommatins. Nevertheless, the exact composition of ommochromes from the eyes of investigated insects requires further examination, e.g., by mass spectrometry.
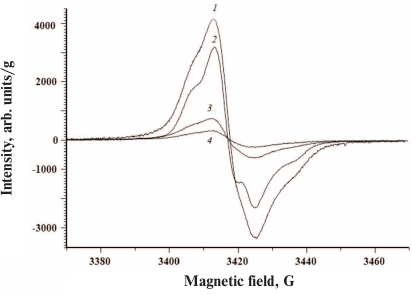
All investigated ommochromes displayed clearly pronounced singlet EPR signal (Fig. 4).
Fig. 4. EPR spectra of ommochromes from the black soldier fly (1), tobacco hornworm butterfly (2), marble cockroach (3), and desert locust (4). The spectra were recorded using dry ommochrome preparations at room temperature.
The parameters of the EPR signals of ommochromes from all five insect species are presented in the Table. All ommochromes have g-factors close to the g-factor of free electron and contained relatively large amounts of paramagnetic centers. The highest concentration of spins per g dry weight was detected in the ommochromes from the black soldier fly and tobacco hornworm butterfly (>1018).
Parameters of EPR signals of insect ommochromes
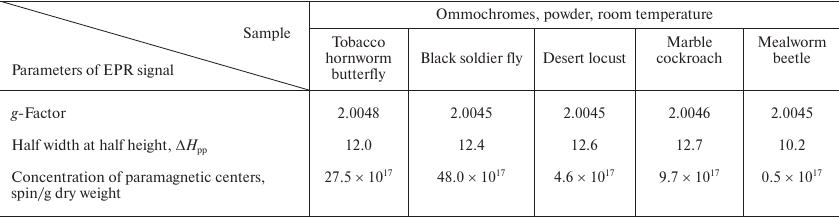
The ommochromes from other insects had the concentration of paramagnetic centers below 1018; furthermore, the spin concentration in the ommochromes from the desert locust and mealworm beetle was more than an order of magnitude lower than in the ommochromes from the black soldier fly. The high concentration of stable free radical centers suggests that ommochromes can act as scavengers of active free radicals. The g-factor value in the range from 2.004 and 2.005 (Table) is characteristic for phenoxy radicals [31]. It is known that phenoxazine intermediates in the composition of ommochrome molecules exhibit a stable EPR signal [32, 33] and likely can be the source of the EPR signals observed in this study. Moreover, it cannot be ruled out that it is precisely phenoxazine that determines the antiradical activity of ommochromes [17]. The EPR signal of the ommochromes was sensitive to illumination by either UV or visible light at the liquid nitrogen temperature (data not shown). The intensity of EPR signal significantly increased upon illumination.
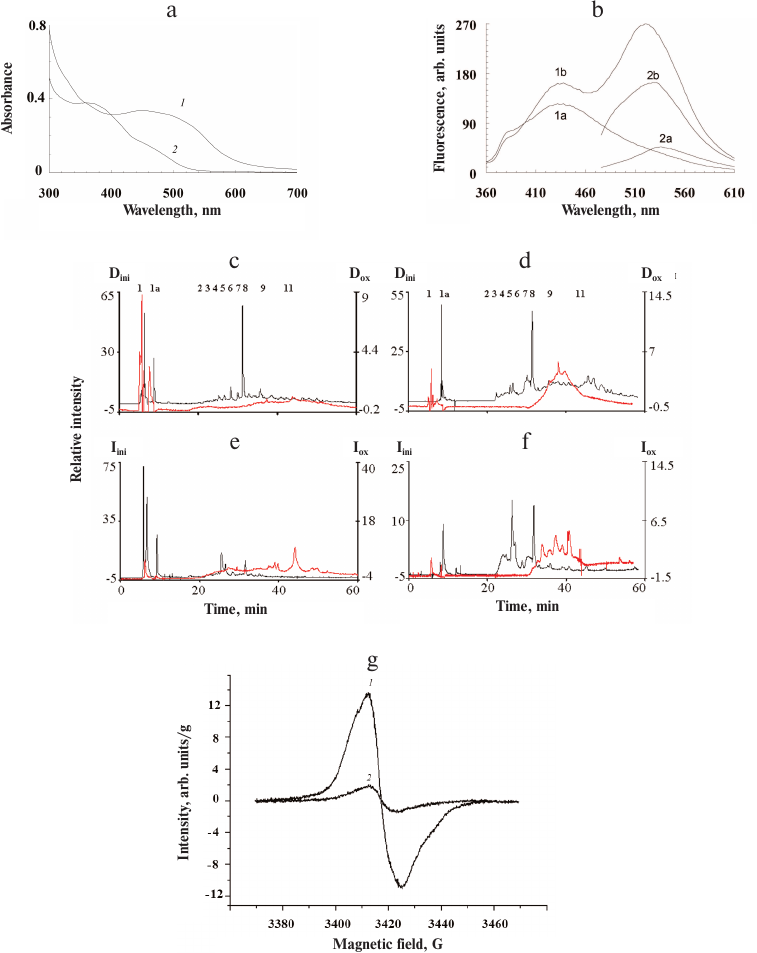
The ommochromes were found to be sensitive to oxidation with hydrogen peroxide and/or potassium superoxide. Oxidation with hydrogen peroxide resulted in the disappearance of the long-wavelength absorption maximum at 430-502 nm (Fig. 5a, curve 1) and emergence of the short-wavelength maximum at 370-390 nm (Fig. 5a, curve 2). This indicated that the isolated ommochromes were predominantly in the reduced state. The reaction with hydrogen peroxide occurs in at least two stages. First, there is transition of ommochromes into the oxidized form, which is likely followed by gradual pigment destruction during prolonged incubation with the oxidizer manifested as further decrease of the pigment absorption in the visible range (Fig. 5a) and significant loss in the number of paramagnetic centers.
Fig. 5. Effect of hydrogen peroxide on insect ommochromes. a) Absorption spectra of ommochromes from the mealworm beetle before (1) and after (2) oxidation with hydrogen peroxide; b) fluorescence spectrum of ommochromes from the mealworm beetle at the excitation wavelengths of 340 nm (1) and 460 nm (2) before (a) and after (b) exposure to hydrogen peroxide; c-f) HPLC analysis of ommochromes from the mealworm beetle before (black) and after (red) exposure to hydrogen peroxide: c) absorption at 380 nm; d) fluorescence at 520 nm with excitation at 380 nm; e) absorption at 490 nm; f) fluorescence at 520 nm with excitation at 460 nm. Dini and Iini, y-axes for absorbance and fluorescence, respectively, before exposure; Dox and Iox, y-axes for absorbance and fluorescence, respectively, after exposure to hydrogen peroxide. g) EPR spectrum of ommochromes from the tobacco hornworm butterfly before (1) and after (2) oxidation with hydrogen peroxide. The spectra of ommochrome suspensions were recorded in 0.1 M K-phosphate buffer at the temperature of liquid nitrogen.
The fluorescent properties of ommochromes changed as a result of oxidation with hydrogen peroxide. As seen in Fig. 5b, the fluorescence intensity of the investigated samples increased significantly following oxidation, mainly in the long-wavelength region (500-560 nm).
HPLC analysis of the ommochromes revealed significant qualitative and quantitative changes in their composition after oxidation with hydrogen peroxide (Fig. 5, c-f). It can be seen that the peaks 2-8 present in the non-oxidized sample almost completely disappeared following sample exposure to hydrogen peroxide. At the same time, the relative content of peaks 9-11 displaying strong fluorescence increases significantly. The reasons for the increase in the ommochrome fluorescence intensity after oxidation with hydrogen peroxide require further investigation.
The EPR signal of the ommochromes was also sensitive to the action of hydrogen peroxide (Fig. 5g, curves 1 and 2). Oxidation of ommochromes with hydrogen peroxide resulted in a sharp drop in the EPR signal and, eventually, complete loss of paramagnetic centers, which was likely associated with the destruction of phenoxazine ring in the structure of ommochrome molecules [17] that initially demonstrated free radical properties. The destruction of Drosophila eye ommochromes by hydrogen peroxide was demonstrated previously [34].
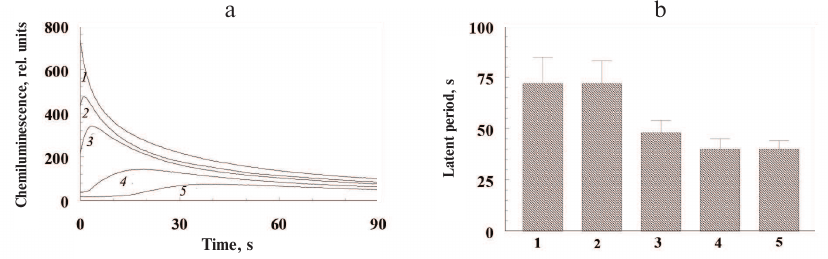
Low concentrations of ommochromes quench luminol chemiluminescence induced by hydrogen peroxide. The kinetics of luminol chemiluminescence in the presence of varying concentrations of ommochromes from the marble cockroach is shown in Fig. 6a. Both the decrease in the amplitude of chemiluminescence signal and increase in the lag period of the development of luminescence maximum were observed in the presence of ommochromes.
Fig. 6. Quenching of luminol chemiluminescence by insect ommochromes. a) Kinetics of luminol chemiluminescence in the presence of different concentrations of ommochromes from the marble cockroach: 1) control; 2-5) 100 µg/ml, 150 µg/ml, 250 µg/ml, and 400 µg/ml ommochromes, respectively. Samples containing buffer solution without the ommochromes served as controls. b) Latent period of luminol chemiluminescence in the presence of different ommochromes at a concentration of 750 µg/ml; bars: 1) black soldier fly; 2) marble cockroach; 3) tobacco hornworm butterfly; 4) mealworm beetle; 5) desert locust. Each bar represents results of four independent measurements. The difference was considered statistically significant at p < 0.05. The incubation medium contained 0.05 M K-phosphate buffer, pH 7.4, 2.0 µM hemoglobin, 100 µM luminol, 100 µM EDTA, and suspension of ommochromes in K-phosphate buffer. The reaction was initiated by adding 100 µM of hydrogen peroxide.
The ommochromes at a concentration of 400 µg/ml (Fig. 6a, curve 5) caused significant inhibition of luminol chemiluminescence. The relative duration of the latent period of the luminol chemiluminescence was determined at the same concentration for all investigated ommochromes (750 µg/ml) in order to compare their antiradical activity (Fig. 6b). The ommochromes from the black soldier fly and marble cockroach (Fig. 6b, bars 1 and 2) demonstrated the highest antiradical activity, while the ommochromes from the mealworm beetle and desert locust (bars 4 and 5) had the lowest one. This result is in agreement with the data on the concentration of stable free radical centers in the ommochromes, which was the lowest in the ommochromes from the mealworm beetle and desert locust. On the other hand, a higher antiradical activity of ommochromes from the marble cockroach (bar 2) in comparison with the activity of ommochromes form the tobacco hornworm butterfly (bar 3) remains unclear, because the concentration of paramagnetic centers in the ommochromes from the tobacco hornworm butterfly is significantly higher than in the ommochromes from the marble cockroach. As mentioned above, the constant of luminol chemiluminescence quenching by the ommochromes from the black soldier fly was relatively high, which implies that these pigments are rather strong antioxidants. It follows from Fig. 6b that the efficiency of luminol chemiluminescence quenching by the ommochromes from the marble cockroach is close to the one by the ommochromes from the black soldier fly, while the efficiencies of chemiluminescence quenching by the ommochromes from the tobacco hornworm butterfly, mealworm beetle, and desert locust are lower, but still comparable to it. It is important to note that the concentration of ommochromes in the eyes of investigated insects is very high. According to our calculations, the content of eye ommochromes reaches 90 mg/ml in the black soldier fly and 45 mg/ml in the desert locust. This is significantly higher that the ommochrome concentration used in our experiments (<1 mg/ml).
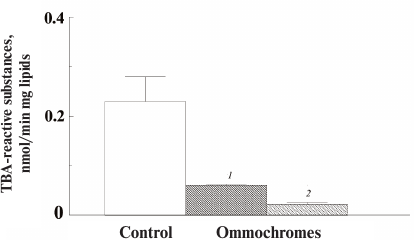
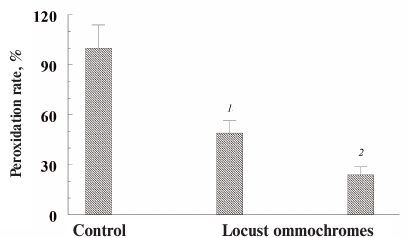
All the investigated ommochromes inhibited peroxidation initiated with various peroxidation systems. The antioxidant activity of ommochromes was determined in three different systems: Fe2+/ascorbate-induced peroxidation of cardiolipin liposomes (Fig. 7); ascorbate-induced peroxidation of outer segments of bovine eye photoreceptors (results not shown); and photoinduced peroxidation of the photoreceptor cell outer segments sensitized by lipofuscin granules from the human retinal pigment epithelium (Fig. 8).
Fig. 7. Inhibitory effect of ommochromes from the black soldier fly (1) and tobacco hornworm butterfly (2) on the Fe2+/ascorbate-induced peroxidation of cardiolipin liposomes. Each bar represents results of three independent measurements. The difference was considered statistically significant at p < 0.05. The incubation medium contained 0.1 M K-phosphate buffer, pH 7.4, 265 µg/ml of cardiolipin liposomes, 0.5 mM ascorbate, and 35 µM Fe2+. The ommochrome concentration was 90 µg/ml; the control did not contain ommochromes.
Fig. 8. Inhibitory effect of ommochromes from the desert locust on the peroxidation of photoreceptor outer segments initiated by visible light in the presence of lipofuscin granules. Each bar represents results of three independent measurements. The difference was considered statistically significant at p < 0.05. The incubation medium contained 0.1 M K-phosphate buffer, pH 7.4, 200 µg/ml photoreceptor outer segment protein, and 5 × 106 granule/ml lipofuscin; ommochrome concentration was 46 (1) and 77 µg/ml (2); control (peroxidation rate in the absence of ommochromes) was accepted as 100%.
In these experiments, we used ommochromes from the black soldier fly, tobacco hornworm butterfly (Fig. 7), and desert locust (Fig. 8). All these ommochromes demonstrated pronounced antioxidant activity by inhibiting peroxidation and photoperoxidation at relatively low concentrations. The antioxidant activity of the insect ommochromes was comparable to the activity of natural melanins [12] and synthetic antioxidants of the oxypyridine series [23]. In our experiments, the ommochromes at a concentration of 0.5 mg/ml caused almost 90% inhibition of free-radical processes. It is important to note that the ommochromes significantly slowed down the photoperoxidation of the photoreceptor cell outer segment sensitized with lipofuscin (Fig. 8). The reaction was inhibited by 70% already at the ommochrome concentration of 0.08 mg/ml (≈0.2 mM), while 0.5 mM mexidol (well-known synthetic antioxidant) inhibited the same reaction by no more than 50% [35]. Hence, it is reasonable to suggest that ommochromes, which are present in the compound eyes of insects in large amounts, could exhibit significant antioxidant activity. The mechanisms of the antioxidant action of ommochromes could be associated with their ability to react with reactive oxygen species and to utilize free radicals [9, 11, 15, 16].
In conclusion, we have developed a relatively simple procedure for the isolation of ommochromes from the insect heads. Based on their physicochemical characteristics, these pigments can be assigned to ommatins [1, 2, 8, 36]. Insects used for feeding animals are cultivated under controlled conditions (both in laboratories and commercially), they can be a good natural source of large quantities of ommochromes. Based on our data, it is possible to isolate more than 4% of dry weight of ommochromes from the biomass wet weight. Due to their biological activity, these natural pigments can be promising pharmacological preparations for prevention and treatment of pathologies associated with the oxidative stress development. The results obtained in this study are important for understanding the mechanisms underlying the biological functions of ommochromes that act as screening and antioxidant pigments in the ommatidia of the compound eyes of invertebrates.
Funding. This work was supported by the Russian Foundation for Basic Research (project No. 19-04-00411).
Conflict of interest. The authors declare no conflict of interest in financial or any other sphere.
Compliance with ethical norms. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
1.Butenandt, A., and Schafer, W. (1962) Recent
Progress in the Chemistry of Natural and Synthetic Coloring Matters and
Related Fields (Gore, T. S., Joshi, B. S., Sunthankar, S. V., and
Tilak, B. D., eds.) Academic Press, NY, USA, pp. 13-34,
doi: 10.1177/004051756303300710.
2.Figon, F., and Casas, J. (2019) Ommochromes in
invertebrates: biochemistry and cell biology, Biol. Rev. Camb.
Philos. Soc., 94, 156-183, doi: 10.1111/brv.12441.
3.Riou, M., and Christides, J.-P. (2010) Cryptic
color change in a crab spider (Misumena vatia): identification
and quantification of precursors and ommochrome pigments by HPLC, J.
Chem. Ecol., 36, 412-423,
doi: 10.1007/s10886-010-9765-7.
4.Stavenga, D. G., Leertouwer, H. L., and Wilts, B.
D. (2014) Coloration principles of nymphaline butterflies – thin
films, melanin, ommochromes and wing scale stacking, J. Exp.
Biol., 217, 2171-2180, doi: 10.1242/jeb.098673.
5.Panettieri, S., Gjinaj, E., John, G., and Lohman,
D. J. (2018) Different ommochrome pigment mixtures enable sexually
dimorphic Batesian mimicry in disjunct populations of the common
palmfly butterfly, Elymnias hypermnestra, PLoS One,
13, e0202465, doi: 10.1371/journal.pone.0202465.
6.Dontsov, A. E., Fedorovich, I. B., Lindström,
M., and Ostrovsky, M. A. (1999) Comparative study of spectral and
antioxidant properties of pigments from the eyes of two Mysis
relicta (Crustacea, Mysidacea) populations, with different light
damage resistance, J. Compar. Physiol. B, 169, 157-164,
doi: 10.1007/s003600050206.
7.Gribakin, F. G. (1981) Mechanisms of
Photoreception in Insects, Nauka, Leningrad, p. 214.
8.Ostrovsky, M. A., Zak, P. P., and Dontsov, A. E.
(2018) Vertebrate eye melanosomes and invertebrate eye ommochromes as
screening cell organelles, Biol. Bul. Russ. Acad. Sci.,
45, 570-579, doi: 10.1134/S0002332918060103.
9.Ostrovsky, M. A., Sakina, N. L., and Dontsov, A. E.
(1987) An antioxidative role of ocular screening pigments, Vis.
Res., 27, 893-899,
doi: 10.1016/0042-6989(87)90005-8.
10.Insausti, T. C., LeGall, M., and Lazzari, C. R.
(2013) Oxidative stress, photodamage and the role of screening pigments
in insect eyes, J. Exp. Biol., 216, 3200-3207,
doi: 10.1242/jeb.082818.
11.Ostrovsky, M. A., and Dontsov, A. E. (2019)
Vertebrate eye melanosomes and invertebrate eye ommochromes as
antioxidant cell organelles, Biol. Bull. Russ. Acad. Sci.,
46, 105-116, doi: 10.1134/S1062359019010084.
12.Ushakova, N., Dontsov, A., Sakina, N., Bastrakov,
A., and Ostrovsky, M. (2019) Antioxidative properties of melanins and
ommochromes from black soldier fly Hermetia illucens,
Biomolecules, 9, 408, doi: 10.3390/biom9090408.
13.Stowe, S. (1983) Phagocytosis of rhabdomeral
membrane by crab photoreceptors, Cell Tissue Res., 234,
463-467, doi: 10.1007/BF00213782.
14.Feldman, T. B., Dontsov, A. E., Yakovleva, M. A.,
Fedorovich, I. B., Lindsrom, M., Donner, K., and Ostrovsky, M. A.
(2008) Comparison of antioxidant systems in the eyes of two Mysis
relicta (Crustacea: Mysidacea) populations, with different light damage
resistance, Sens. Sist., 22, 309-316.
15.Romero, Y., and Martinez, A. (2015) Antiradical
capacity of ommochromes, J. Mol. Model., 21, 220,
doi: 10.1007/s00894-015-2773-3.
16.Zhuravlev, A. V., Zakharov, G. A., Shchegolev, B.
F., and Savvateeva-Popova, E. V. (2016) Antioxidant properties of
kynurenines: density functional theory calculations, PLoS Comput.
Biol., 12, e1005213,
doi: 10.1371/journal.pcbi.1005213.
17.Farmer, L. A., Haidasz, E. A., Griesser, M., and
Pratt, D. A. (2017) Phenoxazine: a privileged scaffold for
radical-trapping antioxidants, J. Org. Chem., 82,
10523-10536, doi: 10.1021/acs.joc.7b02025.
18.Egorov, S. Yu., Krasnovsky, A. A., Dontsov, A.
E., and Ostrovsky, M. A. (1987) Quenching of singlet molecular oxygen
by screening pigments – melanins and ommochromes,
Biofizika, 32, 685-687.
19.Egorov, S. Yu., Babizhaev, M. A., Krasnovsky, A.
A., and Shvedova, A. A. (1987) Photosensitized generation of singlet
molecular oxygen be endogenous photosensitizers from human eye lens,
Biofizika, 32, 169-171.
20.Snytnikova, O. A., Sherin, P. S., Kopylova, L.
V., and Tsentralovich, Yu. P. (2007) Kinetics and mechanism of
reactions of photoexcited kynurenine with some natural compounds,
Russ. Chem. Bull., 56, 732-738,
doi: 10.1007/s11172-007-0109-x.
21.Tsentalovich, Y. P., Snytnikova, O. A., Sherin,
P. S., and Forbes, M. D. (2005) Photochemistry of kynurenine, a
tryptophan metabolite: properties of the triplet state, J. Phys.
Chem. A, 109, 3565-3568, doi: 10.1021/jp045142k.
22.Dontsov, A. E., Ushakova, N. A., Sadykova, V. S.,
and Bastrakov, A. I. (2020) Ommochromes from Hermetia illucens:
isolation, investigation of antioxidant characteristics and
antimicrobial activity, Appl. Biochem. Microbiol., 56,
91-95, doi: 10.1134/S0003683820010044.
23.Smirnov, L. D., Kuznetsov, Yu. V., Proskuryakov,
S. Ya., Skvortsov, V. G., Nosko, T. N., and Dontsov, A. E. (2011)
Antiradical and NO-inhibiting activity of β-hydroxy(ethoxy)
derivatives of nitrous heterocycles, Biofizika, 56,
276-280, doi: 10.1134/S000635091102028X.
24.Dontsov, A. E., Sakina, N. L., and Ostrovsky, M.
A. (2017) Loss of melanin by retinal pigment epithelium cells is
associated with its oxidative destruction in melanolipofuscin granules,
Biochemistry (Moscow), 82, 916-924,
doi: 10.1134/S0006297917080065.
25.Mc Dowell, J. H. (1993) Preparing rod outer
segment membranes, regenerating rhodopsin, and determining rhodopsin
concentration, in: Methods in Neurosciences (Hargrave, P. A.,
ed.) Acad. Press, New York, 15, pp. 123-130,
doi: 10.1016/B978-0-12-185279-5.50013-3.
26.Li, J., Berntsen, B. T., and James, A. A. (1999)
Oxidation of 3-hydroxykynurenine to produce xanthommatin for eye
pigmentation: a major branch pathway of tryptophan catabolism during
pupal development in the yellow fever mosquito, Aedes aegypti,
Bioch. Mol. Biol., 29, 329-338,
doi: 10.1016/s0965-1748(99)00007-7.
27.Teselkin, Yu. Yu., Babenkova, I. V., Lyubitsky,
O. B., Klebanov, G. I., and Vladimirov, Yu. A. (1997) The measurement
of antioxidant activity of blood plasma by the hemoglobin –
hydrogen peroxide – luminol system, Vopr. Med. Khim.,
43, 87-92.
28.Ottolenghi, A. (1959) Interaction of ascorbic
acid and mitochondrial lipids, Arch. Biochem. Biophys.,
7, 355-363, doi: 10.1016/0003-9861(59)90414-X.
29.Figon, F., Munsch, T., Croix, C., Viaud-Massuard,
M.-C., Lanoue, F., and Casas, J. (2019) Biological identification and
localization of uncyclized xanthommatin, a key intermediate in
ommochrome biosynthesis: an in vitro-in vivo study,
bioRxiv Preprint, doi: 10.1101/666529.
30.Martel, R. R., and Law, J. H. (1991) Purification
and properties of an ommochrome-binding protein from the hemolymph of
the tobacco hornworm, Manduca sexta, J. Biol. Chem.,
266, 21392-21398.
31.Bolton, J. R. (1972) Experimental aspects of
biological electron spin resonance studies, in Biological
Application of Electron Spin Resonance (Swartz, H. M., Bolton, J.
R., and Borg, D. C., eds.) Wiley (Interscience), N. Y., USA, p. 11.
32.Lhoste, J.-M., Haug, A., and Ptak, M. (1966)
Electron paramagnetic resonance studies of photoselected triplet
molecules. I. Phenoxazine, J. Chem. Phys., 44, 648-654,
doi: 10.1063/1.1726739.
33.Bolognese, A., Bonomo, R. P., Chillemi, R., and
Sciuto, S. (1990) Oxidation of 3-hydroxykynurenine. An EPR
investigation, J. Heterocyclic Chem., 27, 2207-2208,
doi: 10.1002/jhet.5570270762.
34.Ephrussi, B., and Herold, J. L. (1944) Studies of
eye pigments of drosophila. I. Methods of extraction and quantitative
estimation of the pigment components, Genetics, 39,
148-175.
35.Dontsov, A. E., Koromyslova, A. D., Kuznetsov,
Yu. V., Sakina, N. L., and Ostrovsky, M. A. (2014) Antiradical and
photoprotective activity of oxibiol – a novel water-soluble
heteroaromatic antioxidant, Russ. Chem. Bull., 63,
1159-1163, doi: 10.1007/s11172-014-0565-z.
36.Becker, E. (1942) On the properties, distribution
and the genetic developmental physiological significance of the
pigments of the ommatin and ommin group (ommochromes) in arthropods,
Mol. Gener. Genet., 80, 157-204,
doi: 10.1007/BF01741981.