Levels of Zinc Transporters mRNA Depending on Zinc Status and HIV-1 Tat Induced Inflammation in Muscle (Rhabdomyosarcoma) and Monocyte (THP-1) Cell Lines
Kiran Alluri1,a, Srinivasa Reddy Yathapu2,b, Narendra Babu Kondapalli3,c, Rajkumar Hemalatha3,d, Krishna Madhavan Nair4,e*, and Sudip Ghosh1,f*
1Molecular Biology Division, ICMR-National Institute of Nutrition, 500007 Hyderabad, India2Drug Toxicology Division, ICMR-National Institute of Nutrition, 500007 Hyderabad, India
3Microbiology and Immunology Division, ICMR-National Institute of Nutrition, 500007 Hyderabad, India
4Micronutrient Division, ICMR-National Institute of Nutrition, 500007 Hyderabad, India
* To whom correspondence should be addressed.
Received June 1, 2020; Revised August 27, 2020; Accepted September 15, 2020
Monocytes and muscles demonstrate functionally contrasting behavior under conditions of zinc deficiency with relation to zinc storage system (muscle retain zinc in contrast to monocytes). We aimed to understand the effects of zinc status and HIV-1 Tat mediated inflammation on expression of zinc transporters in these types of cells. Expression of zinc transporters [ZnTs, ZIPs, and metallothionein (MT)] was quantified by qRT-PCR in RD, THP-1 cells separately and in co-cultured THP-1–RD cells. ZnT1 protein expression levels were confirmed by Western blot. Significant increase of MT and ZnT1 mRNA in response to zinc supplementation and decrease during zinc deficiency indicates significance of the genes encoding transporters in maintaining zinc homeostasis in these tissues. In the RD cells ZIP10 exhibited inverse relation to zinc status whereas no correlation was found in the THP-1 cells. Tat-induced inflammation resulted in the significant elevation of MT, IL6, ZIP7, ZIP8, ZIP9 transcripts in the co-cultured RD cells, whereas THP-1 cells demonstrated increased IL-1β levels and reduced levels of ZIP7 and ZIP14. Zinc status and HIV-1Tat induced inflammation appear to influence differential expression of MT, ZnTs, and ZIPs in the muscle and monocyte cells.
KEY WORDS: zinc transporters, ZIPs, ZnTs, co-culture, HIV-1Tat, THP-1, RDDOI: 10.1134/S000629792102005X
Abbreviations: DMEM, Dulbecco’s modified eagle medium; FBS, fetal bovine serum; gp120, glycoprotein120; HIV, Human Immunodeficiency Virus; IL, interleukin; MT, metallothionein; MTF, metal transcription factor; MHC, major histocompatibility complex; Tat, trans-activator of transcription (Tat); TNF, tumour necrosis factor-α; RD, rhabdomyosarcoma cells; TPEN, N,N,N′,N′TetraKis (2-pyridylmethyl) ethylene diamine; ZIPs, Zrt- and Irt-like protein; ZnTs, zinc transporters.
INTRODUCTION
Zinc level remains unchanged in skeletal muscle, skin, and heart whereas it is decreased in bone, monocytes, liver, testis, and plasma during Zn deficiency [1, 2]. Zinc concentrations in lymphocytes, granulocytes, and platelets tend to decrease over 8-12 weeks of zinc deficiency [3]. Plasma Zn concentrations do not change under transient mild zinc deficiency (12-16 µM), but persistent Zn shortage leads to the decrease of plasma zinc concentration [4]. Zn homeostasis is maintained by coordinated regulation of the activities of zinc metal transporters, which belong to two major families: ZnTs (zinc transporters) and ZIPs (Zrt- and Irt-like protein) as well as metallothionein (MT, an intracellular zinc binding protein). ZnTs (ZnT1-ZnT10) belong to the Solute carrier family 30 (SLC) involved in the efflux of intracellular Zn and are encoded by the respective group of genes; while ZIPs (ZIP1-ZIP14) zinc transporters increase intracellular zinc concentrations and are encoded by the SLC39 gene family.
Furthermore, expression of the ZIP influxers changes with age and is regulated post-transcriptionally. It has been reported that zinc status per se influences expression of ZIP1, ZIP2, and ZIP3 in lymphocytes of young adults more strongly in comparison with the elderly [5]. Zinc toxicity is averted by endocytosis of the ZIP4 transporter from the plasma membrane or ubiquitin mediated degradation [6]. When zinc is available, ZIP5 translation is enhanced and is mediated by a conserved stem-loop and two overlapping miRNA seed sites in the 3′-untranslated region [7].
Zinc plays an essential role in the development, maturation and functions of natural killer (NK) and natural killer T (NKT) cells [8]. Mast cell activation in the delayed-type allergic response is known to be mediated by ZnT5 [9]. During bacterial infection/sepsis in mice, ZIP8 is found to be upregulated in response to NF-κB activation. The ZIP8-mediated influx of zinc inhibits IκB kinase (IKK) and thus suppresses inflammation [10]. Similarly, ZIP9 is also known to increase intracellular zinc levels leading to activation of the protein kinase B (PKB/AKT), extracellular signal-regulated kinase (ERK) phosphorylation, and B cell receptor (BCR) [11]. Studies have shown that higher concentrations of Zn inhibit human immunodeficiency virus (HIV-1) transcription and production of new infectious virions, whereas Zn co-supplementation with Zidovudine (AZT) reduces opportunistic infections and diarrhea-related morbidity in the HIV-infected children [12-15]. It is well established that HIV is a zinc-dependent retrovirus, wherein Zn stimulates viral enzyme – “integrase”, thereby facilitating integration of the viral DNA into the host DNA [16, 17]. In fact, chemotherapeutic efficiency of some aromatic C-nitroso compounds is based on their ability to eject zinc from the zinc-finger motifs of HIV-1 nucleocapsid thus preventing viral infection [18].
It has been reported that HIV-1 proteins such as glycoprotein (gp120), negative factor (Nef), trans-activator of transcription (Tat), and regulator of expression of virion proteins (Rev) can induce muscle wasting, cataracts, nephropathy, skin lesions and immune deficiencies [19, 20]. Tat-induced programmed cell death leads to depletion of lymphocytes in the HIV-infected persons [21]. In contrast, Tat has mitogenic activity against mammary and amniotic epithelial cells and is responsible for mother-to-child transmission of the virus [22]. Interestingly, zinc is essential for the activity of Tat protein required for replication of HIV [23]. It is known that Tat stimulates fluid secretion from the serosal to the luminal side of enterocytes, whereas zinc prevents such activity [24].
Tat has an inhibitory effect on major histocompatibility complex (MHC) class I and II and induces expression of the pro-inflammatory cytokines and thus modulates immune responses to HIV-1 infection [25, 26]. In addition, it has been well documented that the development of cachexia or slim disease in the HIV/AIDS patients is facilitated by the MHC-I-restricted cytotoxic T-cell mediated polymyositis [27, 28]. Cachexia is further aggravated by the pro-inflammatory cytokines like interleukin (IL)-1β and tumour necrosis factor (TNF)-α secreted by the HIV-infected monocytes/macrophages [29]. The origin of skeletal muscle wasting in the HIV/AIDS patients could be attributed to the changes in the circulatory levels of cytokines and myostatin [30, 31].
Considering all these data, current study aimed to understand the role of zinc status and inflammation in the zinc releasing (monocytes) and zinc storing (muscle) cells. We hypothesized that zinc transporters are responsible for the differential response of these cells to zinc deficiency and inflammation. Therefore, the experiments were performed using representative tissue specific cell lines THP-1 (Monocytes) and human rhabdomyosarcoma (RD) muscle cells investigating their response to exogenous zinc supplementation, zinc depletion with N,N,N′,N′TetraKis (2-pyridylmethyl) ethylene diamine (TPEN-intracellular zinc chelator), and HIV-1 Tat peptide-induced inflammation.
MATERIALS AND METHODS
Chemicals. Cell culture chemicals, Dulbecco’s Modified Eagle Medium (DMEM), Roswell Park Memorial Institute Medium (RPMI)-1640, fetal bovine serum (FBS), glutamax, antibiotic–antimycotic, Poly-L-lysine, Zinquin ethyl ester, dimethyl sulfoxide (DMSO) and TPEN ethylene diamine were procured from Sigma-Aldrich (USA). HIV-1 Consensus B Tat Peptide Pool (Cat:12706) is a gratis from the NIH-AIDS Reagent Program, USA.
Source of cell lines. A leukemic human monocytic cell line THP-1 is a generous gift from CSIR-IICT, Hyderabad, India; whereas RD cell line was purchased from American type culture collection (ATCC), Manassas, USA. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2 and medium was replaced every other day. THP-1 and RD cells were cultured in RPMI-1640 and DMEM medium, respectively, supplemented with 10% FBS and 1% of each glutamax and antibiotic-antimycotic. Cells were maintained at logarithmic growth phase and experiments were performed when the cells attained ~70-80% confluence. Culture media did not contain zinc except in FBS.
Zinc and TPEN treatment of THP-1 and RD cells. Cells were washed with phosphate buffered saline (PBS) to remove any excess of zinc from the medium. THP-1 and RD cells were treated either with 25 µM zinc as zinc sulfate in complex with 25 µM bovine serum albumin (BSA, Sigma- Aldrich) [32] or with 5 µM TPEN (an intracellular zinc chelator) to induce zinc deficiency assessed based on trypan blue viability for 4 h in the serum-free medium. Then the THP-1 cells were fixed on coverslips coated with 0.1 mg/ml poly-L-lysine. All experiments were conducted three times as independent experiments.
Zinquin ethyl ester uptake in RD and THP-1 cell lines. As described in the previous section, the cells were incubated either with 25 µM zinc as zinc sulfate or 5 µM TPEN for 4 h in the serum-free medium. Cells were washed with PBS and then incubated with 10 µM zinc-specific fluorophore “Zinquin ethyl ester” for 30 min [33]. The fluorescent zinc-zinquin complex was visualized by recording fluorescence emission at 490 nm excited at 370 nm using fluorescence microscope with a GFP filter (EVOS FL-Life Technologies, USA).
Quantification of intracellular zinc. THP-1 cell suspension (5 × 106/ml) and trypsinized RD cells were incubated with 10 µM Zinquin ethyl ester at 37°C for 30 min [34]. Cells were washed three times with PBS and resuspended in PBS, fluorescence emission of 2-ml samples (5 × 106 cells/ml) in cuvettes was measured at 490 nm with excitation at 370 nm using a Jasco FP-6500 spectrofluorometer (Japan).
Western Blotting for ZnT1 transporter. Cells were lysed with RIPA buffer containing protease inhibitor cocktail and protein concentrations in the supernatant were assessed using a micro-BCA kit method. Proteins (50 µg) were separated using 10% SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad, USA). After blocking with a 5% skim milk powder solution, the membrane was incubated with an anti-ZnT1 antibody overnight at 4°C followed by washing and incubation with an HRP-conjugated IgG secondary antibody for 1 h. The blots were developed using an ECL detection kit (Bio-Rad) and images were acquired with a G-box imaging system (Syngene, USA). β-Actin and GAPDH were used as references to control for equal protein loading of THP-1 and RD cells, respectively.
Co-cultivation of RD and THP-1 cells for HIV-1Tat challenge. To understand the role of Tat-induced inflammation on the cell-cell interaction, co-culture of RD and THP-1 cells was performed. Briefly, RD cells were plated at a density of 0.9 × 106/ml, the medium was replaced with fresh DMEM after 24 h. Monocytes were seeded onto the RD monolayer at 1 : 1 ratio and after addition of Tat (100 ng/ml), incubated for 8 h [35]. A THP-1 cell suspension seeded onto RD cells in a similar way was used as a control for co-cultured cells. After incubation, the THP-1 suspended cells were aspirated and washed out with PBS and the adherent RD cells were washed extensively with PBS to remove suspended THP-1 cells. These washed cells were used for quantification of the expression of MT, ZnTs, ZIPs, IL-6, IL-1β using qRT-PCR.
Quantitative real-time PCR (qRT-PCR). Total RNA was isolated from cells using Trizol (Life Technologies) following the manufacturer’s protocol. One microgram of RNA was used for cDNA synthesis using a Verso cDNA synthesis kit (ThermoFisher Scientific, USA) and qRT-PCR was carried out using 2× SYBR green (Takara Bio, Japan) and gene-specific sets of primers (Table S1 in the Supplement) in a C1000 thermal cycler, (BIO RAD CFX96, Hercules, USA). All assays were run in duplicates. Amplified PCR products were also analyzed on an ethidium bromide-stained agarose gel to confirm amplification of a single product. GAPDH and β-actin were used for normalization for RD and THP-1 cells, respectively. PCR products of all the samples were also subjected to the melting curve analysis to verify a single amplification product and corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. Relative mRNA expression was quantified according to the 2–ΔΔ CT method.
Intracellular cytokine staining (ICS) for TNFα. The cytokine TNF-α response to Tat peptide pool was investigated in immune cells, i.e., THP-1 cells only. Briefly, the co-cultured THP-1 cells were exposed to Tat (100 ng/ml), then GolgiStopTM (BD Biosciences, USA) was added and incubated for not more than 12h. The reaction was terminated by spinning down the cells and washing with Sheath fluid (BD FACS Flow). Later cells were re-suspended in 250 µl of cytofix/cytoperm solution in dark at room temperature for 20 min. Cells were washed twice with wash buffer and incubated with PE-cy7 TNF-α antibody (BD Biosciences) at room temperature in dark for 30 min to determine the membrane bound TNF-α levels. Unbound antibody was washed twice with Perm/Wash buffer and re-suspended in 400 µl of wash buffer and data acquired on a flow cytometer (BD FACS Aria-II, USA), and analyzed using the Flow Jo 10.7 software (Ashland, USA).
MHC-I in inflammation. Co-cultured RD and THP-1 cell were stained with an anti-HLA-ABC-FITC (Miltenyi Biotec, Germany) or an isotype control (REA Control FITC, Miltenyi Biotec) for 30 min at 4°C as per manufacturer’s instructions. Cells were analyzed with flow cytometry as explained above.
RESULTS
Zinc homeostasis is maintained by regulation of the tissue specific zinc transporter expression. However, a detailed gene expression studies in the functionally contrasting tissues are unavailable. In the current study, the intracellular zinc levels were quantified using the fluorescent dye by fluorescence microscopy and spectro-fluorimetry in cells. The expression patterns of MTs, ZnTs, ZIPs were analyzed by quantitative RealTime PCR (qRT-PCR).
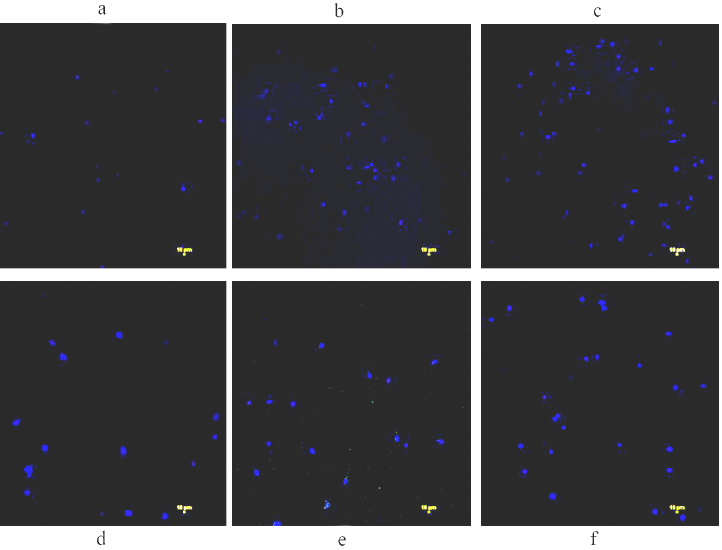
Intracellular Zn levels in zinc supplementation and depletion treatments. The cells cultured with zinc and TPEN for 4 h showed that Zinquin ethyl ester fluorescence in both THP-1 (Fig. 1, a-c) and RD (Fig. 1, d-f) compared with respective controls, suggesting that internalization of zinquin can be used for quantification of zinc in these cells. Subsequently, mean zinc levels in both cell types were significantly (p < 0.05) elevated during zinc supplementation (7.92 ± 0.10 nM in THP-1 and 13.5 ± 0.44 nM in RD) as compared to the control cells (1.97 ± 0.103 nM in THP-1 and 5.78 ± 0.3 nM in RD). While a significant (p < 0.05) drop in the zinc levels was noted in these cells treated with TPEN (0.94 ± 0.06 nM in THP-1 and 3.98 ± 0.45 nM in RD) compared to the control (Table S2 in the Supplement). Thus, both THP-1 and RD cells are responding to Zn status, these observations are in agreement with our previous studies of the bone cells [36].
Fig. 1. Assessment of intracellular zinc in RD and THP-1 cells. Cells were treated either with 25 µM zinc or 5 µM TPEN for 4 h. Cells were washed with PBS to remove extracellular dye and images were captured using λex 359 nm/λem 485 nm recording blue fluorescence of the Zinc – Zinquin ethyl ester complex. Untreated THP-1 cells were used as control (untreated) (a); zinc treated 25 µM (b) or TPEN treated 5 µM (c). Similarly, RD cells as control (untreated) (d); zinc treated 25 µM (e); TPEN treated 5 µM (f) cells were incubated with Zinquin ethyl ester 10 µM for 30 min.
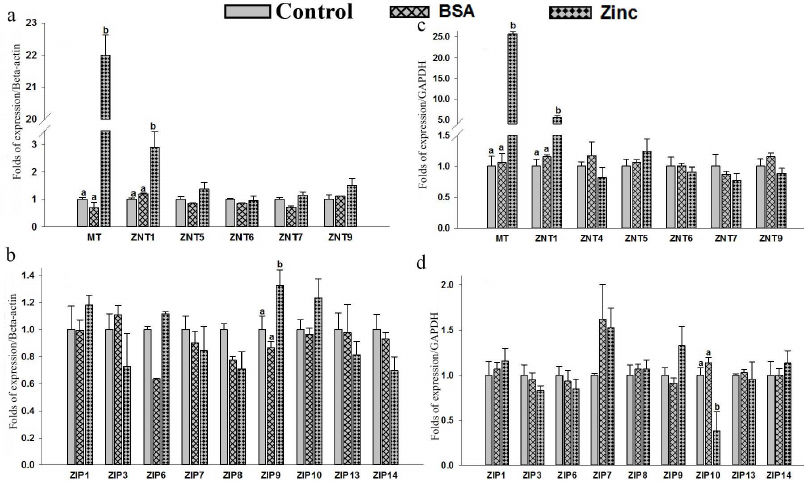
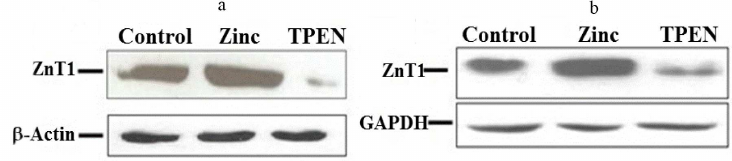
Expression of MT, hZnTs, and hZIPs in response to zinc supplementation. Expression levels of MT, hZnTs, and hZIPs of THP-1 were measured by qRT-PCR in cells supplemented with 25 µM of zinc (ZnSO4). A significant (p < 0.05) up-regulation (22 folds) of the metallothionein (MT) mRNA levels in THP-1 cells suggests strong response to zinc supplementation (Fig. 2a; Table S3 in the Supplement). Similarly, a significant (p < 0.05) 2.9-fold elevation of ZnT1 mRNA levels were observed in the THP-1 cells (Fig. 2a). Furthermore, this observation was corroborated with the increased ZnT1 expression observed at the protein level (Fig. 3a). Expression of ZIP9 was also significantly (p < 0.05) increased (1.3-fold) in the THP-1 cells supplemented with exogenous zinc as compared to control (Fig. 2b).
Similarly, a significant (p < 0.05) increase in the expression of metallothionein (25-fold) and ZnT1 (5.6-fold) (p < 0.05) in the RD cells suggested positive response to the zinc excess by the RD cells (Fig. 2c). Furthermore, quantification of the protein with Western blot confirmed upregulation of ZnT1 in these cells (Fig. 3b). Among the various ZIPs, the ZIP10 expression was found to be decreased (0.3-fold) significantly as compared to the untreated control (Fig. 2d).
Supplementation of zinc had no significant effect on the expression of other ZnTs (ZnT4 – ZnT7 and ZnT9) and ZIPs (ZIP1, ZIP3, ZIP6 – ZIP9, ZIP13 and ZIP14). The expression of ZnT2, ZnT3, ZnT8, ZnT10, ZIP2, ZIP4, ZIP5 and ZIP12 were undetectable in the THP-1 and RD cells.
Fig. 2. Expression of zinc transporter genes in THP-1 and RD cells. Total RNA was isolated and cDNA was synthesized. Relative mRNA quantification was carried out by qPCR using SYBR-Green intercalating dye. mRNA was normalized to mRNA β-actin/GAPDH. THP-1: MT, hZnTs mRNA levels (a) and hZIPs mRNA levels (b). RD cells: MT, hZnTs mRNA levels (c) and hZIPs mRNA levels (d). Cells treated with 25 µM zinc for 4 h. Bars represent mean ± SE of n = 3 from three independent experiments. Different superscripts present significance of differences at p < 0.05. Significance of differences between treated cells versus control was analyzed using Analysis of variance (ANOVA) with post hoc multiple comparison by Dunnett’s t-test.
Fig. 3. Western-blot analysis of total level of the ZnT1 transporter protein in THP-1 and RD cells following zinc supplementation or depletion for 4 h. a) THP-1 cells and (b) RD cells treated with either zinc or TPEN (zinc chelator). CTR = control, zinc = supplemented with 25 µM zinc, TPEN = Zinc depletion by intra cellular zinc chelator. GAPDH was used as internal reference control in RD and β-actin – for THP-1 cells for normalizing protein loading in cells.
To the best of our knowledge, responses of the expression kinetics of most zinc transporters in human muscles (a major Zn reservoir) to zinc fluctuations and inflammatory conditions have not been reported so far. Expression of the metallothionein (MT), a zinc storage protein, is dependent on zinc levels and could affect a number of cellular processes [37, 38]. A significant increase in MT levels (22-25 folds in THP-1 and RD) in response to zinc supplementation was noted in both THP-1 and RD cells. These observations are in line with the previous in vitro studies on the THP-1 response to zinc [39].
The metal transcription factor-1 (MTF) plays a critical role in transcriptional activation of both MT and ZnT1 in response to metals [40, 41]. Therefore, as expected, both MT and ZnT1 levels were significantly elevated in the THP-1 and RD cells under zinc sufficiency condition, which corroborated earlier reports on in vitro (THP-1 cells) and in vivo studies (small intestine, liver, and kidney) [42-44]. Despite functionally contrasting roles in maintaining zinc homeostasis in the representative tissues (THP-1-monocytes and RD cells-muscles), their common mode of regulation via MTF-1 mediated activation in the presence or absence of zinc [40, 41] suggest common manner of sensing the whole-body zinc status. Therefore, we speculate that ZnT1 is one of the predominant zinc transporters regulating intracellular zinc levels in the muscle cells as well as in monocytes, which is in agreement with its ubiquitous expression as an efflux transporter across the plasma membrane in many different types of cells [45]. The non-responsiveness of certain ZnTs and ZIPs to Zn supplementation could be attributed to their role in Zn homeostasis, for instance, ZnT4 and ZnT6 transport Zn from intracellular compartments to periphery, when Zn concentrations are quite high [46]. Accordingly, ZIP4 significantly respond to Zn deficiency rather than to its sufficiency [46].
Furthermore, observations of the current study are in line with the findings reported by Overbeck et al. of low expression of ZnT4 and no expression of ZnT2, ZnT3, and ZnT8 in the THP-1 cells cultured with zinc sulphate [43]. While no significant changes were noted in the ZnT5 mRNA levels in THP-1 cells in the presence of zinc, it was found to be decreased in a study carried out with THP-1 [43]. The MTF-1 is known to exert dual action of either activation or depression depending on Zn status. It down regulates the expression of ZIP10 under Zn sufficiency and up regulates under Zn deficiency [47]. However, when the cells were exposed to zinc, expression of ZIP10 did not change significantly in the THP-1, while it was decreased in the RD cells, which was corroborated by the observation reported by Ryu et al. (2008) [48]. The repression of ZIP10 under Zn sufficiency could be attributed to the regulation mechanism involving MTF-1 binding to metal response elements (MREs), by which MTF-1 pauses Pol II transcription [46].
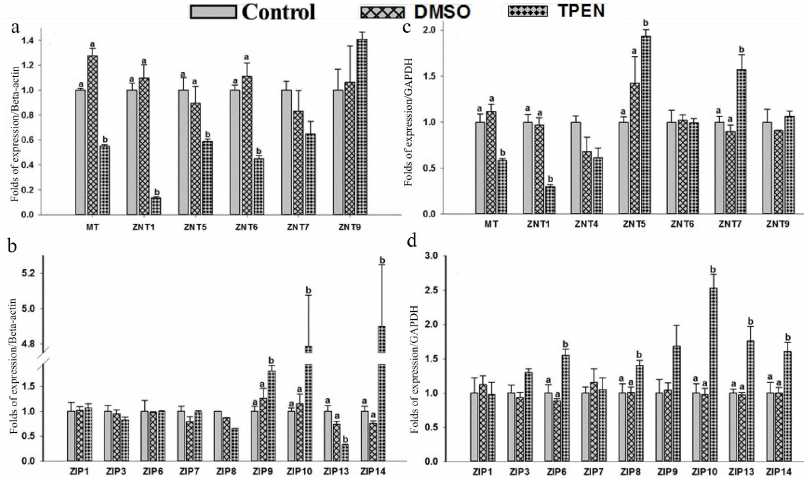
Effect of Zinc depletion on expression of MT, ZnTs, and ZIPs in THP-1 and RD cells. Depletion of intracellular zinc by TPEN caused significant down-regulation of MT (0.55-fold), ZnT1 (0.13-fold), ZnT5 (0.58-fold), and ZnT6 (0.44-fold) expression in the THP-1 cells compared to the control (Fig. 4a). This mRNA synthesis correlated with the protein levels of ZnT1 determined with Western blotting (Fig. 3a). The findings of down-regulation of MT, ZnT1, ZnT5 and ZnT6 corroborated earlier reports on Zn depleted THP-1 cells [39, 49]. At the same time, a significant up-regulation of ZIP9 (1.81-fold), ZIP10 (4.7-fold) and ZIP14 (~4.9-fold) along with down regulation of ZIP13 levels (0.3-fold) were observed in the THP-1 cells under condition of depleted zinc (Fig. 4b). However, Zn depletion has no significant effect on ZnT4, ZnT7, ZnT9 and ZIP1, ZIP3, ZIP6, ZIP7, ZIP8 expression. On the other hand, ZnT2, ZnT3, ZnT8, ZnT10, ZIP2, ZIP4, ZIP5, and ZIP12 expression was undetectable in the Zn-depleted THP-1 cells. Regarding ZIP2, our findings contradicted to the earlier reports stating the prominent role of ZIP2 in the Zn-deficient THP-1 cells, which could be due to the experimental differences of using either 5 µM TPEN 18-h exposure or 16 µM TPEN 4-h exposure to mimic intracellular zinc depletion [35, 39].
Fig. 4. Gene expression of zinc transporter in response to TPEN treatment in THP-1 and RD cells. Total RNA was isolated and cDNA was synthesized. Relative mRNA quantification was carried out by qPCR using SYBR-Green intercalating dye. mRNA was normalized to mRNA B-actin/GAPDH. Expression of mRNA levels in cells treated with 5 µM TPEN. THP-1: MT, hZnTs mRNA levels (a) and hZIPs mRNA levels (b). RD cells: MT, hZnTs mRNA levels (c) and hZIPs mRNA levels (d). Bars represent mean values ± SE (n =3) from three independent experiments. Different superscripts indicate significance of differences at p < 0.05. Significance of differences between treated cells versus control was analyzed using Analysis of variance (ANOVA) with post hoc multiple comparison by Dunnetts t-test.
Similarly, RD cells responded to zinc deficiency by significant down-regulation of MT (0.5-fold) and ZnT1 (0.3-fold) (Fig. 4c); which was confirmed by the protein expression levels (Fig. 3b). However, under this condition expression of ZnT5, ZnT7, ZIP6, ZIP8, ZIP10, ZIP13, and ZIP14 was upregulated significantly by 1.9-, 1.5-, 1.5-, 1.4-, 2.5-, 1.7-, and 1.6-fold, respectively as compared to control (Fig. 4, c and d). In contrast to the THP-1 cells, upregulation of ZnT5 was observed in the RD cells under Zn deficiency. Nonetheless, Zn depletion has no noticeable effect on the expression pattern of other ZnTs (ZnT4, ZnT6, ZnT9) and ZIPs (ZIP1, ZIP3, ZIP7). Though statistically insignificant, a slight up-regulation of ZIP9 was noted. Expression of eight of the zinc transporters was not detected (ZnT2, ZnT3, ZnT8, ZnT10 and ZIP2, ZIP4, ZIP5, ZIP12). Hence, the ZIPs are differentially expressed in the THP-1 and RD cells in response to Zn deficiency.
It was reported that MTF-1’s dual action is responsible for down-regulation of ZIP10 under zinc abundance and its up-regulation under zinc-deficiency conditions [47]. Our findings on ZIP10 in the THP-1 and RD cells are in agreement with these observations; 4.7- and 2.5-fold higher expression of ZIP10 were noted in the THP-1 and RD cells, respectively, under zinc depletion [46, 47]. Among the various efflux and influx zinc transporters MT, ZnT1, and ZIP 10 appear to follow similar mechanistic regulation under the conditions of excess and depletion of zinc in monocytes and muscle cells. However, we found opposing expression patterns of ZIP13 and ZIP14 in the THP-1 and RD cells during zinc depletion. Gene knockout experiments in mice targeting ZIP13 and ZIP 14 revealed that these Zn transporters regulated Zn-mediated signalling processes, thus such impairment caused abnormalities in systemic growth and bone homeostasis [46]. These observations allowed elucidating the role of MTF-1 in regulation of MT, ZnT1 and ZIPs (ZIP10, ZIP13, and ZIP14) in response to Zn status in THP-1 and RD cells.
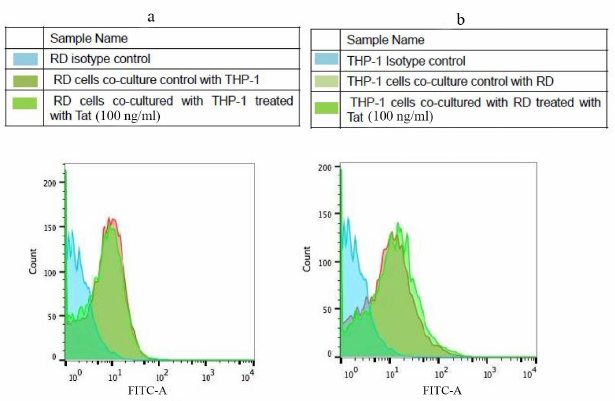
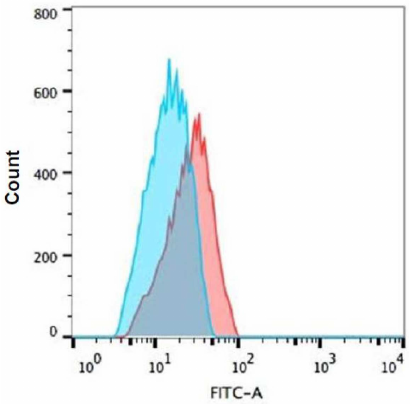
Effect of Tat-induced inflammation on MHC-1 and intracellular TNF-α. Tat induced (100 ng/ml) effect on MHC-1 was assessed in the THP-1 and RD co-cultured cells using FACS. It was found that the expression levels of MHC-I remained unchanged in both types of cells in the presence of Tat as compared to the respective controls (Fig. 5). However, the intracellular TNF-α levels were significantly increased (~3.6 fold) in the THP-1 co-cultured cells in the presence of Tat (100 ng/ml) as compared to the untreated controls (Fig. 6).
Fig. 5. Expression of MHC-1 in Tat-treated RD and THP-1 cells. Co-cultured cells (a) RD muscle (b) THP-1 monocytes cells were treated with HIV-1Tat – peptide pools for 8 h and MHC-I expression was evaluated with flow cytometry. Representative histograms are shown with cells cultured in the medium alone (control histogram) overlaid with the histogram for treated cells (Tat treated histogram colors).
Fig. 6. Expression of TNF-α in Tat-treated THP-1 cells. Co-cultured THP-1 cells were treated with 100 ng/ml Tat peptide pools for 8 h and TNF-α expression was evaluated by intracellular cytokine TNF-α staining with flow cytometry. Representative histograms are shown: blue histogram – control and red – Tat treated cells. Shift indicates increased TNF-α expression. Cells cultured in the medium alone control (blue histogram) overlaid with the histogram for Tat-treated cells (red line).
To understand the effect of inflammation on zinc transporters, we co-culture the THP-1 and RD muscle cells to simulate a cross talk between the representative tissues and exposed them to HIV-Tat peptides for 8 h. Previous reports on the inflammatory response of THP-1 cells to Tat protein (100 nM for 12 h) have shown elevated levels of TNF-α [50]. Similarly, our findings on the co-cultured THP-1 cells exposed to Tat have resulted in significant elevation of the intracellular TNF-α level.
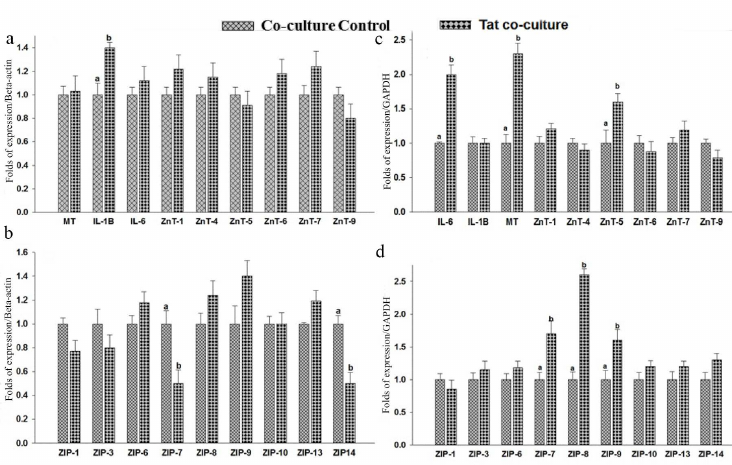
Co-culture of THP-1 and RD cells. Effect of Tat on zinc transporter expression in the co-cultured THP-1 cells. THP-1 cells co-cultured with RD cells have shown an inflammatory response to Tat by elevating the IL-1β levels 1.4-fold (Fig. 7a). Whereas no difference was observed in the IL-6 levels as compared to the THP-1 control co-cultured cells. Studies of macrophages treated with Tat protein for 4h has showed increased IL6 and IL-1β levels [51]. In the current study, a significant expression of IL-1β was noted in Tat exposed THP-1 cells but no such effect was observed for the IL6 levels. Tat-induced inflammation resulted in the significant (0.5-fold, p < 0.05) down-regulation of zinc transporters ZIP7 and ZIP14 in the co-cultured THP 1 cells as compared to the untreated controls (Fig. 7b). These observations are in agreement with the response of mouse Dendritic Cells (DCs) to Lipopolysaccharide (LPS), where ZIP6 and ZIP10 are down-regulated, while ZnT1, ZnT4, and ZnT6 are up-regulated causing a net decrease in the cytosolic Zn content [52]. Though statistically insignificant, an increase in the ZIP9 levels was noted in the Tat exposed THP-1 co-cultured cells. However, there were no significant changes in MT, ZnT1, ZnT4 – ZnT7, ZnT9, ZIP1, ZIP3, ZIP6, ZIP8, ZIP10, and ZIP13 levels of the Tat exposed THP-1 cells co-cultured with RD cells.
Fig. 7. Gene expression in co-cultured THP-1 and RD cells. RNA was isolated and relative mRNA quantification was carried out by real-time SYBR GREEN-PCR. mRNA levels were normalized to mRNA β-actin/GAPDH. Levels of mRNA expression in the cells exposed to Tat (100 ng/ml) for 8 h. THP-1: hZnTs levels (a) and hZIPs expression levels (b). RD cells: hZnTs levels (c) and hZIPs expression levels (d). Bars represent mean ± SE (n = 3). Different superscripts indicate significance of differences at p < 0.05 by paired independent test.
Similarly, Tat effect on the expression of ZIPs (the influxers of zinc), suggests a significant reduction in expression of ZIP7 and ZIP14 in co-cultured THP-1 cells, these findings were in line with the previous reports on the effect of inflammation on lung epithelial cells [53]. On the contrary, infection/inflammation upregulates ZIP14 through IL-6 /IL-1β resulting in hypozincemia by transporting zinc into liver [54, 55]. Data available in the literature also suggest that stimulation of inflammation by LPS in the dendritic cells leads to ZIP6 downregulation resulting in upregulation of MHC-II [56]. Therefore, our observation of unaltered MHC-I levels could be due to the unchanged levels of ZIP6 in response to Tat. Furthermore, it is evident that DCs that overexpress ZIP6 fail to activate the antigen-specific CD4+ Th cells [52].
ZIP14 is known to be located on the plasma membrane and imports zinc into the cytoplasm from the extracellular medium. In the current study, ZIP14 expression was found to be downregulated in response to HIV Tat protein. We speculate that ZIP14 down regulations may retain the serum zinc levels which could facilitate HIV virus infection. On the other hand, lack of the sufficient levels of zinc in THP-1 triggers apoptosis [57, 58].
Effect of Tat on zinc transporter expression in the co-cultured RD cells. The RD cells co-cultured with THP-1 cells have shown a significant (p < 0.05) elevation of IL-6 levels (2-fold) as compared to the untreated control, suggesting a proinflammatory response to Tat (Fig. 7c). Whereas, the IL-1β levels were comparable between the RD cells co-cultured either with or without Tat peptides. The metallothionein (MT) response to Tat exposure resulted in the significant up-regulation (2.3-fold) in the co-cultured RD cells as compared to untreated control. There were no significant changes in the IL-1β levels of the Tat-exposed co-cultured RD cells. It was reported that Tat-induced inflammatory responses were tissue specific and differential expression was confirmed by the IL6 expression pattern in PBMCs (minimal), THP-1 (mild), and astrocytes (strong) in response to Tat peptide exposure [59].
Similarly, the levels of zinc transporters such as ZnT5 (1.6-fold), ZIP7 (1.7-fold), ZIP8 (2.6-fold), and ZIP9 (1.6-fold) were significantly elevated in the co-cultured RD cells in response to Tat treatment (Fig. 7d). Whereas, there were no significant changes in the levels of other ZnTs (ZnT1, ZnT4, ZnT6, ZnT7, ZnT9) and ZIPs (ZIP1, ZIP3, ZIP6, ZIP10, ZIP13, ZIP14) in Tat exposed RD cells when co-cultured with THP-1 cells (Fig. 7, c and d).
Tat effect on the expression of ZIPs, the influxers of zinc, suggest a significant reduction in the expression of ZIP7 and ZIP14 in the co-cultured THP-1 cells, whereas increase in the levels of ZIP7, ZIP8, and ZIP9 was noted in RD cells. It is likely that the muscle cells dictate the monocyte to downregulate ZIPs so that they can acquire more zinc for storage. However, lack of the data on transcriptional regulation of MTF-1 and apoptotic markers does not allow us to validate this suggestion.
CONCLUSIONS
It appears that two functionally contrasting cells RD (muscle) and THP-1 (monocytes) maintain intracellular zinc homeostasis by the coordinated regulation of MT and zinc transporters and act in concert to modulate transient changes of zinc muffling. ZIP10 demonstrated differential regulation in muscles as it was found to be down regulated by the excess zinc but remained unchanged in the THP-1 cells under these conditions. HIV-1Tat mediated inflammation downregulated some of the zinc transporters, i.e., ZIP7 and ZIP14 especially in the THP-1 cells when co-cultured with RD cells, whereas ZIPs were elevated in the RD muscle cells. This may indicate redirection of zinc into muscle, which in this case could act as a passive reservoir of zinc. The decline in the number of monocytes in HIV/AIDS patients is probably mediated through lowering intracellular zinc levels thus triggering apoptosis of monocytes. Though the results of the current study were based on the cell line models, they may help us to understand the distinct responses to zinc status and inflammation.
Acknowledgments. We acknowledge CSIR-New Delhi, India, for financial assistance to Mr. Kiran Alluri for JRF and SRF (09/484/(0050)/2012-EMR-1). Authors gratefully acknowledge the support of Dr. B. Dinesh Kumar, Scientist G & Head-DTRC for extending the facility and chemicals/reagents from ICMR-Taskforce Project Ref. No. HIV/62/47/2016-ECD-II.
Funding. This research was financially supported by the Indian Council of Medical Research (intramural grants to KMN and grant to SG (5/9/1137/2014-NUT).
Ethics declarations. The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies involving human participants or animals performed by any of the authors.
Supplementary information. The online version contains supplementary material available at https://doi.org/10.1134/S000629792102005X.
REFERENCES
1.Kambe, T., Tsuji, T., Hashimoto, A., and Itsumura,
N. (2015) The physiological, biochemical, and molecular roles of zinc
transporters in zinc homeostasis and metabolism, Physiol. Rev.,
95, 749-784.
2.Jackson, M. (1989) Physiology of Zinc: General
Aspects, Zinc in Human Biology, pp. 1-14.
3.Beck, F. W., Kaplan, J., Fine, N., Handschu, W.,
and Prasad, A. S. (1997) Decreased expression of CD73
(ecto-5′-nucleotidase) in the CD8+ subset is
associated with zinc deficiency in human patients, J. Lab. Clin.
Med., 130, 147-156.
4.Prasad, A. S. (2008) Zinc in human health: effect
of zinc on immune cells, Mol. Med., 14, 353-357.
5.Giacconi, R., Malavolta, M., Costarelli, L., Busco,
F., Galeazzi, R., et al. (2012) Comparison of intracellular zinc
signals in nonadherent lymphocytes from young-adult and elderly donors:
role of zinc transporters (Zip family) and proinflammatory cytokines,
J. Nutr. Biochem., 23, 1256-1263.
6.Mao, X., Kim, B. E., Wang, F., Eide, D. J., and
Petris, M. J. (2007) A histidine-rich cluster mediates the
ubiquitination and degradation of the human zinc transporter, hZIP4,
and protects against zinc cytotoxicity, J. Biol. Chem.,
282, 6992-7000.
7.Weaver, B. P., and Andrews, G. K. (2012) Regulation
of zinc-responsive Slc39a5 (Zip5) translation is mediated by conserved
elements in the 3′-untranslated region, Biometals,
25, 319-335.
8.Mocchegiani, E., Giacconi, R., Cipriano, C., and
Malavolta, M. (2009) NK and NKT cells in aging and longevity: role of
zinc and metallothioneins, J. Clin. Immunol., 29,
416-425.
9.Nishida, K., Hasegawa, A., Nakae, S., Oboki, K.,
Saito, H., et al. (2009) Zinc transporter Znt5/Slc30a5 is required for
the mast cell-mediated delayed-type allergic reaction but not the
immediate-type reaction, J. Exp. Med., 206,
1351-1364.
10.Liu, M.-J., Bao, S., Gálvez-Peralta, M.,
Pyle, C. J., Rudawsky, A. C., et al. (2013) The zinc transporter
SLC39A8 is a negative feedback regulator of NF-κB through
zinc-mediated inhibition of IKK, Cell Rep., 3, 386.
11.Taniguchi, M., Fukunaka, A., Hagihara, M.,
Watanabe, K., Kamino, S., Kambe, T., Enomoto, S., and Hiromura, M.
(2013) Essential role of the zinc transporter ZIP9/SLC39A9 in
regulating the activations of Akt and Erk in B-cell receptor signaling
pathway in DT40 cells, PLoS One, 8, e58022.
12.Haraguchi, Y., Sakurai, H., Hussain, S., Anner,
B. M., and Hoshino, H. (1999) Inhibition of HIV-1 infection by zinc
group metal compounds, Antiviral Res., 43, 123-133.
13.Mocchegiani, E., and Muzzioli, M. (2000)
Therapeutic application of zinc in human immunodeficiency virus against
opportunistic infections, J. Nutr., 130, 1424S-1431S.
14.Bobat, R., Coovadia, H., Stephen, C., Naidoo, K.
L., McKerrow, N., Black, R. E., and Moss, W. J. (2005) Safety and
efficacy of zinc supplementation for children with HIV-1 infection in
South Africa: a randomised double-blind placebo-controlled trial,
Lancet, 366, 1862-1867.
15.Baum, M. K., Lai, S., Sales, S., Page, J. B., and
Campa, A. (2010) Randomized, controlled clinical trial of zinc
supplementation to prevent immunological failure in HIV-infected
adults, Clin. Infect. Dis., 50, 1653-1660.
16.Tang, A. M., Graham, N. M., Kirby, A. J., McCall,
L. D., Willett, W. C., and Saah, A. J. (1993) dietary micronutrient
intake and risk of progression to Acquired Immunodeficiency Syndrome
(AIDS) in Human Immunodeficiency Virus Type 1 (HlV-1)-infected
homosexual men, Am. J. Epidemiol., 138, 937-951.
17.Lee, S. P., and Han, M. K. (1996) Zinc stimulates
Mg2+-dependent 3′-processing activity of human
immunodeficiency virus type 1 integrase in vitro,
Biochemistry, 35, 3837-3844.
18.Rice, W. G., Schaeffer, C. A., Harten, B.,
Villinger, F., South, T. L., et al. (1993) Inhibition of HIV-1
infectivity by zinc-ejecting aromatic C-nitroso compounds,
Nature, 361, 473-475.
19.Reid, W., Sadowska, M., Denaro, F., Rao, S.,
Foulke, J., et al. (2001) An HIV-1 transgenic rat that develops
HIV-related pathology and immunologic dysfunction, Proc. Natl. Acad.
Sci. USA, 98, 9271-9276.
20.Reid, W., Abdelwahab, S., Sadowska, M., Huso, D.,
Neal, A., et al. (2004) HIV-1 transgenic rats develop T cell
abnormalities, Virology, 321, 111-119.
21.Purvis, S. F., Jacobberger, J. W., Sramkoski, R.
M., Patki, A. H., and Lederman, M. M. (1995) HIV type 1 Tat protein
induces apoptosis and death in Jurkat cells, AIDS Res. Hum.
Retroviruses, 11, 443-450.
22.Bettaccini, A. A., Baj, A., Accolla, R. S.,
Basolo, F., and Toniolo, A. Q. (2005) Proliferative activity of
extracellular HIV-1 Tat protein in human epithelial cells: expression
profile of pathogenetically relevant genes, BMC Microbiol.,
5, 20.
23.Frankel, A. D., Bredt, D. S., and Pabo, C. O.
(1988) Tat protein from human immunodeficiency virus forms a
metal-linked dimer, Science, 240, 70-73.
24.Canani, R. B., Ruotolo, S., Buccigrossi, V.,
Passariello, A., Porcaro, F., Siani, M. C., and Guarino, A. (2007) Zinc
fights diarrhoea in HIV-1-infected children: in-vitro evidence to link
clinical data and pathophysiological mechanism, AIDS, 21,
108-110.
25.Li, J. C., Yim, H. C., and Lau, A. S. (2010) Role
of HIV-1 Tat in AIDS pathogenesis: its effects on cytokine
dysregulation and contributions to the pathogenesis of opportunistic
infection, AIDS, 24, 1609-1623.
26.Matsui, M., Warburton, R. J., Cogswell, P. C.,
Baldwin, A. S., Jr., and Frelinger, J. A. (1996) Effects of HIV-1 Tat
on expression of HLA class I molecules, J. Acquir. Immune Defic.
Syndr. Hum. Retrovirol., 11, 233-240.
27.Gherardi, R. K. (1994) Skeletal muscle
involvement in HIV-infected patients, Neuropathol. Appl.
Neurobiol., 20, 232-237.
28.Illa, I., Nath, A., and Dalakas, M. (1991)
Immunocytochemical and virological characteristics of HIV-associated
inflammatory myopathies: similarities with seronegative polymyositis,
Ann. Neurol., 29, 474-481.
29.Belec, L., Meillet, D., Hernvann, A., Gresenguet,
G., and Gherardi, R. (1994) Differential elevation of circulating
interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6 in
AIDS associated cachectic states, Clin. Diagn. Lab. Immunol.,
1, 117-120.
30.Llovera, M., Garcia-Martinez, C., Agell, N.,
Lopez-Soriano, F. J., Authier, F. J., Gherardi, R. K., and Argiles, J.
M. (1998) Ubiquitin and proteasome gene expression is increased in
skeletal muscle of slim AIDS patients, Int. J. Mol. Med.,
2, 69-73.
31.Gonzalez-Cadavid, N. F., Taylor, W. E.,
Yarasheski, K., Sinha-Hikim, I., Ma, K., et al. (1998) Organization of
the human myostatin gene and expression in healthy men and HIV-infected
men with muscle wasting, Proc. Natl. Acad. Sci. USA, 95,
14938-14943.
32.Tibaduiza, E. C., and Bobilya, D. J. (1996) Zinc
transport across an endothelium includes vesicular cotransport with
albumin, J. Cell. Physiol., 167, 539-547.
33.Sreenivasulu, K., Raghu, P., and Nair, K. M.
(2010) Polyphenol-rich beverages enhance zinc uptake and
metallothionein expression in Caco-2 cells, J. Food Sci.,
75, H123-H128.
34.Coyle, P., Zalewski, P. D., Philcox, J. C.,
Forbes, I. J., Ward, A. D., et al. (1994) Measurement of zinc in
hepatocytes by using a fluorescent probe, zinquin: relationship to
metallothionein and intracellular zinc, Biochem. J., 303,
781-786.
35.Chen, L., Frister, A., Wang, S., Ludwig, A.,
Behr, H., et al. (2009) Interaction of vascular smooth muscle cells and
monocytes by soluble factors synergistically enhances interleukin-6 and
MCP-1 production, Am. J. Physiol. Heart Circ. Physiol.,
296, H987-H996.
36.Alluri, K., Nair, K. P., Kotturu, S. K., and
Ghosh, S. (2020) Transcriptional regulation of zinc transporters in
human osteogenic sarcoma (Saos-2) cells to zinc supplementation and
zinc depletion, Biol. Trace Elem. Res., 194, 360-367.
37.Davis, S. R., and Cousins, R. J. (2000)
Metallothionein expression in animals: a physiological perspective on
function, J. Nutr., 130, 1085-1088.
38.Alluri, K., Nair, K. P., and Ghosh, S. (2019)
Differential expression of zinc transporters in functionally
contrasting tissues involved in zinc homeostasis, Nucleosides
Nucleotides Nucleic Acids, 18, 1-5.
39.Cao, J., Bobo, J. A., Liuzzi, J. P., and Cousins,
R. J. (2001) Effects of intracellular zinc depletion on metallothionein
and ZIP2 transporter expression and apoptosis, J. Leukoc. Biol.,
70, 559-566.
40.Andrews, G. K. (2000) Regulation of
metallothionein gene expression by oxidative stress and metal ions,
Biochem. Pharmacol., 59, 95-104.
41.Langmade, S. J., Ravindra, R., Daniels, P. J.,
and Andrews, G. K. (2000) The transcription factor MTF-1 mediates metal
regulation of the mouse ZnT1 gene, J. Biol. Chem., 275,
34803-34809.
42.Cousins, R. J., Blanchard, R. K., Popp, M. P.,
Liu, L., Cao, J., et al. (2003) A global view of the selectivity of
zinc deprivation and excess on genes expressed in human THP-1
mononuclear cells, Proc. Natl. Acad. Sci. USA, 100,
6952-6957.
43.Overbeck, S., Uciechowski, P., Ackland, M. L.,
Ford, D., and Rink, L. (2008) Intracellular zinc homeostasis in
leukocyte subsets is regulated by different expression of zinc
exporters ZnT-1 to ZnT-9, J. Leuk. Biol., 83,
368-380.
44.Liuzzi, J. P., Blanchard, R. K., and Cousins, R.
J. (2001) Differential regulation of zinc transporter 1, 2, and 4 mRNA
expression by dietary zinc in rats, J. Nut., 131,
46-52.
45.Palmiter, R. D., and Findley, S. D. (1995)
Cloning and functional characterization of a mammalian zinc transporter
that confers resistance to zinc, EMBO J., 14,
639-649.
46.Hara, T., Takeda, T. A., Takagishi, T., Fukue,
K., Kambe, T., and Fukada, T. (2017) Physiological roles of zinc
transporters: molecular and genetic importance in zinc homeostasis,
J. Physiol. Sci., 67, 283-301.
47.Lichten, L. A., Ryu, M.-S., Guo, L., Embury, J.,
and Cousins, R. J. (2011) MTF-1-mediated repression of the zinc
transporter Zip10 is alleviated by zinc restriction, PLoS One,
6, e21526.
48.Ryu, M.-S., Lichten, L. A., Liuzzi, J. P., and
Cousins, R. J. (2008) Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8),
and Zip10 (Slc39a10) in mouse red blood cells are differentially
regulated during erythroid development and by dietary zinc deficiency,
J. Nut., 138, 2076-2083.
49.Hamon, R., Homan, C. C., Tran, H. B., Mukaro, V.
R., Lester, S. E., et al. (2014) Zinc and zinc transporters in
macrophages and their roles in efferocytosis in COPD, PLoS One,
9, e110056.
50.Chen, P., Mayne, M., Power, C., and Nath, A.
(1997) The Tat protein of HIV-1 induces Tumor Necrosis Factor-α
production implications for HIV-1-associated neurological diseases,
J. Biol. Chem., 272, 22385-22388.
51.Nath, A., Conant, K., Chen, P., Scott, C., and
Major, E. O. (1999) Transient exposure to HIV-1 Tat protein results in
cytokine production in macrophages and astrocytes A hit and run
phenomenon, J. Biol. Chem., 274, 17098-17102.
52.Hojyo, S., and Fukada, T. J. (2016) Roles of zinc
signaling in the immune system, Immunol Res., 2016,
6762343, doi: 10.1155/2016/6762343.
53.Lang, C. J., Murgia, C., Leong, M., Tan, L.-W.,
Perozzi, G., et al. (2006) Anti-inflammatory effects of zinc and
alterations in zinc transporter mRNA in mouse models of allergic
inflammation, Am. J. Physiol. Lung Cell. Mol. Physiol.,
292, L577-L584.
54.Liuzzi, J. P., Lichten, L. A., Rivera, S.,
Blanchard, R. K., Aydemir, T. B., et al. (2005) Interleukin-6 regulates
the zinc transporter Zip14 in liver and contributes to the hypozincemia
of the acute-phase response, Proc. Natl. Acad. Sci. USA,
102, 6843-6848.
55.Lichten, L. A., Liuzzi, J. P., and Cousins, R. J.
(2009) Interleukin-1β contributes via nitric oxide to the
upregulation and functional activity of the zinc transporter Zip14
(Slc39a14) in murine hepatocytes, Am. J. Physiol. Gastroint. Liver
Physiol., 296, G860-G867.
56.Kitamura, H., Morikawa, H., Kamon, H., Iguchi,
M., Hojyo, S., et al. (2006) Toll-like receptor-mediated regulation of
zinc homeostasis influences dendritic cell function, Nat.
Immunol., 7, 971-977.
57.Li, C. J., Friedman, D. J., Wang, C., Metelev,
V., and Pardee, A. B. (1995) Induction of apoptosis in uninfected
lymphocytes by HIV-1 Tat protein, Science, 268,
429-431.
58.Chen, D., Wang, M., Zhou, S., and Zhou, Q. (2002)
HIV-1 Tat targets microtubules to induce apoptosis, a process promoted
by the pro-apoptotic Bcl-2 relative Bim, EMBO J., 21,
6801-6810.
59.Joshi, P. C., and Guidot, D. M. (2011) HIV-1
transgene expression in rats induces differential expression of tumor
necrosis factor alpha and zinc transporters in the liver and the lung,
AIDS Res. Ther., 8, 36.
Supplementary Tables S1-S3 (PDF)