Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers
Abeer M. Abd_El-Hameed1,a, Ahmed I. Yousef2,b, Sanaa M. Abd_El-Twab2,c, Ahmed A._G. El-Shahawy3,d, and Adel Abdel-Moneim2,e*
1Chemistry Department, Faculty of Science, Taibah University, 30002 Al-Madinah Al-Munawarah, Saudi Arabia2Molecular Physiology Division, Faculty of Science, Beni-Suef University, 62511 Beni-Suef, Egypt
3Materials Science and Nanotechnology Department, Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University, 62511 Beni-Suef, Egypt
* To whom correspondence should be addressed.
Received June 15, 2020; Revised September 3, 2020; Accepted September 9, 2020
Polydatin (PD) has a broad range of pharmacological activities; however, its effects on diabetic liver damage are poorly studies. This work is aimed to explore possible protective effects of polydatin-loaded chitosan nanoparticles (PD-CSNPs) or PD against liver damage associated with diabetes. Diabetes was induced in rats using nicotinamide/streptozotocin treatment. Diabetic rats were then divided into six groups: normal control rats, diabetic control rats, and rats orally treated with PD, PD-CSNPs, equivalent unloaded CSNPs, or metformin daily for 4 weeks. Treatment with PD and PD-CSNPs significantly reduced the blood glucose content, lipid peroxidation in the liver, and activities of serum transaminases and carbohydrate metabolism enzymes (including succinate dehydrogenase and pyruvate kinase); by contrast, liver glycogen content, glutathione concentration, and activities of the antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase, and glucose-6-phosphate dehydrogenase) were markedly increased compared with the control diabetic rats. Furthermore, expression of the tumor necrosis factor α and interleukin-1β mRNAs was significantly downregulated, while expression of glucose transporter 2 and glucokinase mRNAs was strongly upregulated vs. control diabetic rats. We concluded that PD-CSNPs and PD ameliorate diabetic liver damage by modulating glucose transporter 2 expression, affecting the activity of carbohydrate metabolism enzymes, and suppressing oxidative stress and inflammation, PD-CSNPs being more efficient than PD, probably due to higher bioavailability and prolonged release.
KEY WORDS: diabetic liver damage, polydatin-loaded chitosan nanoparticles, carbohydrate metabolism enzymes, glucose transporter 2, oxidative stressDOI: 10.1134/S0006297921020061
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; DM, diabetes mellitus; FBG, fasting blood glucose; G6PD, glucose-6-phosphate dehydrogenase; GK, glucokinase; GLUT2, glucose transporter 2; GPx, glutathione peroxidase; GSH, reduced glutathione; IL-1β, interleukin 1β; MDA, malondialdehyde; NA, nicotinamide; PD, polydatin; PD-CSNPs, polydatin-loaded chitosan nanoparticles; PK, pyruvate kinase; ROS, reactive oxygen species; SDH, succinate dehydrogenase; SOD, superoxide dismutase; STZ, streptozotocin; TNF-α, tumor necrosis factor α.
INTRODUCTION
Diabetes mellitus (DM) is a complex, prevalent, and serious health problem characterized by chronic hyperglycemia resulting from the insulin deficiency and/or its effects [1]. DM is accompanied by the dysfunction and damage of organs, which eventually leads to numerous dangerous complications and high morbidity and mortality among diabetic patients [1, 2]. In the liver, which is one of the affected primary organs, DM causes long-term metabolic dysfunctions, leading to tissue damage and promoting progression of various disorders, such as nonalcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma [3]. Liver damage is widely prevalent among diabetics; 75% of type 2 diabetic patients have non-alcoholic fatty liver disease [4].
Uncontrolled hyperglycemia associated with DM induces oxidative damage, triggers inflammation pathways, and leads to the initiation and progression of liver disease [5]. Lucchesi et al. [6] reported that hyperglycemia induces oxidative stress in the liver, which is manifested as an increase in the reactive oxygen species (ROS) content and reduction in the expression of antioxidant markers. High ROS levels could be generated by oxidative phosphorylation in the mitochondria or via other pathways, such as glucose autooxidation, non-enzymatic glycation, protein kinase C (PKC) activation, and hexosamine and sorbitol pathways [5, 7]. Inflammation could be another essential mechanism of liver damage in diabetic individuals. The expression levels of tumor necrosis factor α (TNF-α) and its receptor TNF-R1 are elevated in the diabetic state, resulting in the upregulation of the inducible nitric oxide synthase (iNOS) expression and NO production in the liver [8]. This increase in the content of pro-inflammatory cytokines amplifies the negative effects of glucotoxicity and leads to mitochondrial dysfunction, oxidative stress, and liver damage [3, 8].
In recent decades, the search for alternative therapies, including effective and safe anti-diabetic agents from plants, has attracted a considerable attention [9]. Polydatin (PD, also known as pieceid or 3,4′,5-trihydroxystilbene-3-β-d-glucoside) was originally extracted from the roots and rhizome of the Chinese herb Polygonum cuspidatum, which is traditionally used for the treatment of fever, pain, cough, and hypertension. PD was also found to be a potent detoxifier for cholestatic liver injury [10]. PD displays the antioxidant activity by regulating ROS production and mitochondrial function and produces the anti-inflammatory effect by reducing the generation of pro-inflammatory cytokines [11]. Despite these promising pharmacological properties, the clinical application of PD is limited due to its low bioavailability because of chemical instability in aqueous alkaline media, poor solubility in water, and pronounced first-pass metabolism [12]. Several research groups are aiming to solve these problems through development of the delivery systems that would protect PD from the degradation, increase the water solubility of the loaded drug, target PD to specific sites, and provide continuous release patterns [13]. The current study was designed to investigate the ameliorating activity of PD-loaded chitosan nanoparticles (PD-CSNPs) and PD on the diabetes-associated liver damage with a focus on the effect of these agents on the glucose metabolism enzymes, expression of glucose transporter 2 (GLUT2), oxidative stress, and expression of pro-inflammatory markers.
MATERIALS AND METHODS
Chemicals. Polydatin, nicotinamide (NA), and streptozotocin (STZ) were from Sigma-Aldrich (USA). Metformin (MET) was from Merck KGaA (Germany). PD-CSNPs were synthesized by the modified ionic gelation method [14] and characterized as described in the recent study of Abdel-Moneim et al. [15]. All other reagents and materials were obtained from standard commercial suppliers.
Animals. Male Wistar albino rats (120-140 g) were purchased from the Holding Company for Biological Products and Vaccines (VACSERA, Egypt). Animals were housed in well-aerated cages at normal atmospheric conditions and normal 12-h light/dark cycle. Experimental animals were managed according to the guidance of the Institutional Animal Care and Use Committee (IACUC) of the Beni-Suef University (IACUC Permit Number: BSU-FS-2018-8).
Induction of the NA/STZ model of diabetes in rats. STZ (50 mg/kg body weight) was dissolved in cold citrate buffer (pH 4.5) and immediately injected intraperitoneally in the overnight-fasted rats 15 min after NA injection (110 mg/kg body weight intraperitoneally; prepared in normal physiological saline) [16]. One-week post-injection, diabetic rats with fasting blood glucose (FBG) level ≥200 mg/dl were included in the study.
Experimental design. Experimental animals were divided into the following six groups (n = 6 per group): normal control rats, diabetic control rats, and diabetic rats given PD (D + PD, 50 mg/kg body weight), PD-CSNPs (D + PD-CSNPs, equivalent to 50 mg/kg body weight PD), unloaded CSNPs (D + CSNPs), and standard oral hypoglycemic agent metformin (D + MET, 100 mg/kg weight). All treatments were administered daily by gastric intubation; the dose was adjusted every week according to changes in the body weight.
Biochemical assays. On the day before the sacrifice (after 4 weeks of treatment), fasting blood samples were taken from the lateral tail vein of overnight-fasted rats (8-10 h). Sera were separated and used for spectrophotometric glucose assay using a reagent kit from Spinreact Co. (Spain). Liver glycogen content was determined by the method of Seifter et al. [17].
The activities of serum aspartate transaminase (AST) and alanine transaminase (ALT) were estimated using reagent kits from Biosystems Company (Spain). The activities of serum succinate dehydrogenase (SDH) and pyruvate kinase (PK) were determined colorimetrically using reagent kits from BioVision Incorporated (Milpitase, USA). The total serum protein and the albumin concentration were estimated using reagent kits from Spinreact Company and Human Diagnostics (Germany), respectively, according to the manufacturer’s instructions.
Liver tissue was homogenized (10%) in normal saline; the homogenate was centrifuged for at 3000 rpm for 10 min, and the supernatant was collected. Liver homogenate was assayed for lipid peroxidation (LPO) [malondialdehyde (MDA) assay], glucose-6-phosphate dehydrogenase (G6PD), reduced glutathione (GSH), and activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) according to the manufacturer’s instructions of the kits purchased from Biodiagnostic (Egypt).
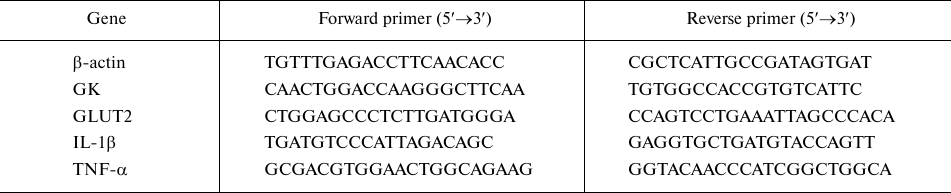
Quantitative PCR. Total RNA was isolated from the liver tissue using a Qiagen tissue extraction kit (USA) according to the manufacturer’s instructions. The concentration and the purity (A260/A280 ratio) of the isolated RNA were evaluated spectrophotometrically using a dual-wavelength Beckman (USA). Total RNA (0.5-2 µg) was used to generate cDNA with a High-capacity cDNA reverse transcription kit (Fermentas, USA) according to the manufacturer’s protocols. Real-time qPCR amplification and analysis were conducted with an Applied Biosystems StepOne™ real-time PCR system (USA) using the provided software (version 3.1). The primer annealing temperatures were optimized for the used primer sets (Table 1). Relative quantification was performed using the ΔΔCt method with the Applied Biosystem software. The RQ is the fold change compared with the normal control.
Table 1. Primer pairs used for qPCR
Histological analysis. Five animals per group were used for the histological analysis of liver tissue. Briefly, a small piece of liver was fixed in 10% neutral buffered formalin for 24 h, dehydrated with an ascending series of ethanol solutions (70%, 95%, and 100%), embedded in paraffin, sectioned at 4-5 µm thickness using a microtome, and stained with hematoxylin-eosin. Five fields in each slide were examined using a light microscope [18].
Statistical analysis. The data were analyzed using SPSS version 20 for Windows (SPSS Inc, USA). One-way analysis of variance (ANOVA) was performed to compare the experimental groups, followed by the least significant difference for multiple comparisons test. The differences were considered significant at p < 0.05.
RESULTS
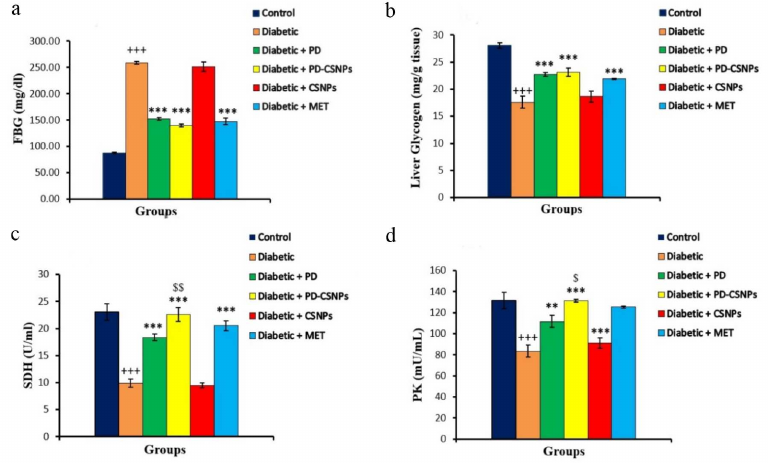
The effects of PD and PD-CSNPs on carbohydrate metabolism. One of the direct manifestations of changes in the carbohydrate hemostasis is alteration in the activity of enzymes involved in carbohydrate metabolism. Figure 1 shows that treatment with PD and PD-CSNPs lowered (p < 0.001) the FBG level, while the activities of serum SDH and PK were markedly increased compared to those in the diabetic control rats. Treatment with PD and PD-CSNPs also increased liver glycogen (p < 0.001). PD-CSNPs produced a more pronounced effect compared with free PD. The effect of PD and PD-CSNPs was comparable to the beneficial effects of Met in the treated vs. diabetic control rats (Fig. 1).
Fig. 1. Effect of PD and PD-CSNPs on the blood glucose, liver glycogen, and activities of key carbohydrate metabolism enzymes. Values are expressed as mean ± standard error. +++, p < 0.001 vs. normal control group; **, p < 0.01; , p < 0.001 vs. diabetic group, $, p < 0.05; $$, p < 0.01 vs. PD-treated diabetic rats (diabetic + PD).
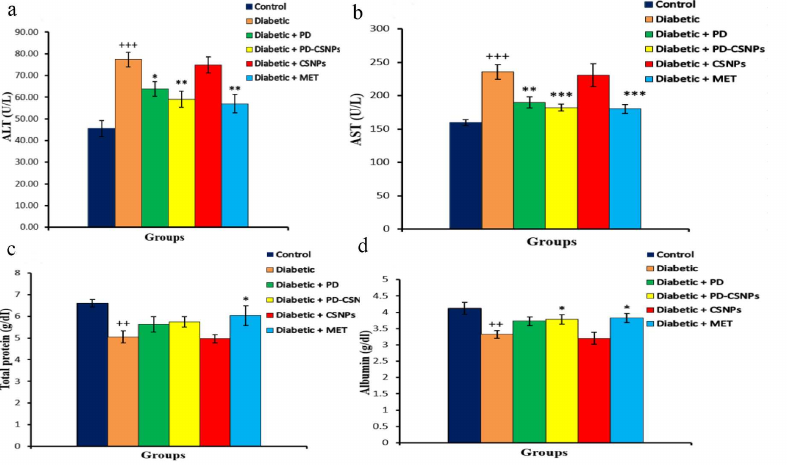
The effects of PD and PD-CSNPs on the activity of transaminases and protein profile. Increased activities of serum AST and ALT are common biochemical markers of liver damage. We observed an increase in both AST and ALT activities in the serum of diabetic rats compared with the normal control rats (p < 0.001). Administration of PD or PD-CSNPs caused a noticeable reduction in the AST and ALT activities in the treated rats. The total serum protein was decreased in the diabetic group vs. the normal control group (p < 0.01), but was significantly elevated (p < 0.05) in the Met-treated group compared with the diabetic control rats. The serum level of albumin also showed a significant decrease vs. the normal control group (p < 0.01), but was markedly increased (p < 0.05) after the treatment with PD-CSNPs or Met (Fig. 2).
Fig. 2. Effect of PD and PD-CSNPs on the hepatic enzymes and protein profile in diabetic rats. Values are expressed as mean ± standard error; ++, p < 0.01 and +++, p < 0.001 vs. normal control group; *, p < 0.05, **, p < 0.01, and , p < 0.001 vs. diabetic group.
The effects of PD and PD-CSNPs on the oxidative stress and antioxidant biomarkers in the liver. Compared with the normal control rats, there was a notable increase (p < 0.001) in the LPO marker MDA in the diabetic rats, as well as the decrease (p < 0.05) in the GSH content and activities of SOD, CAT, GPx, and G6PD in the diabetic rats vs. the normal control group. As predicted, treatment with PD or PD-CSNPs elevated the GSH content and activities of SOD, GPx, CAT, and G6PD simultaneously causing a decrease in the MDA production compared with the diabetic control rats. Therefore, PD, PD-CSNPs, and Met can significantly relieve the oxidative stress and increase expression of the antioxidant markers in the liver tissues of diabetic rats (Table 2).
Table 2. Effect of PD and PD-CSNPs on
oxidative stress and antioxidant biomarkers in the liver of diabetic
rats
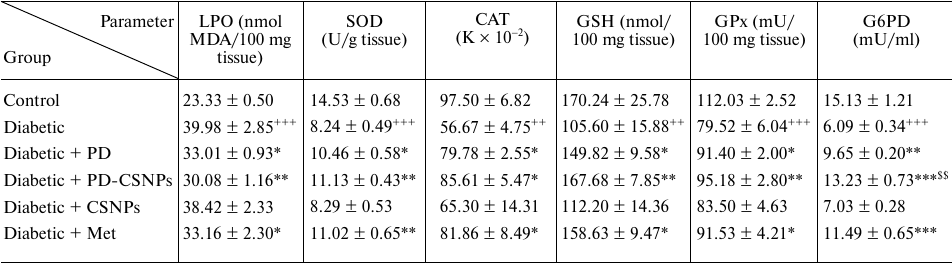
Notes. Values are expressed as mean ± standard error;
++, p < 0.01 and +++, p <
0.001 vs. normal control group; *, p < 0.05, **, p
< 0.01, and , p < 0.001 vs. diabetic group; $$,
p < 0.01 vs. diabetic + PD group.
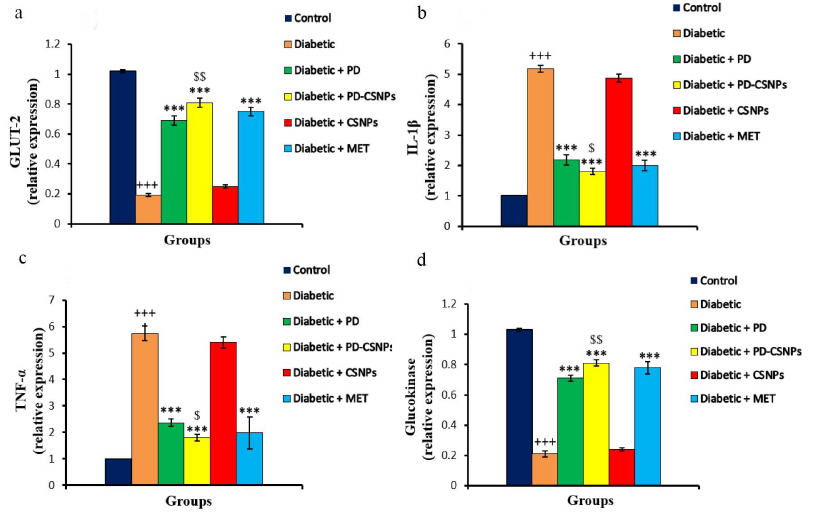
The effects of PD and PD-CSNPs on GLUT2, glucokinase (GK), TNF-α, and interleukin 1β (IL-1 β) expression. Expression of the GLUT2 and GK mRNAs in the liver of diabetic control rats was reduced compared to the normal control group (p < 0.001). Treatment with PD or PD-CSNPs elevated GLUT2 and GK expression levels vs. the diabetic control rats (p < 0.001). Notably, PD-CSNPs produced a more pronounced positive effect compared to PD only (p < 0.01) (Fig. 3).
Fig. 3. Effect of PD and PD-CSNPs on the expression of GLUT2, GK, TNF-α, and IL-1β. Values are expressed as mean ± standard error; +++, p < 0.001 vs. normal control group; , p < 0.001 vs. diabetic group; $, p < 0.05 and $$, p < 0.01 vs. PD-treated diabetic group (diabetic + PD).
The levels of mRNAs for the inflammatory cytokines TNF-α and IL-1β were upregulated in the liver tissues of the diabetic control rats (p < 0.001) vs. the normal control rats. Treatment with PD or PD-CSNPs downregulated TNF-α expression (p < 0.001) relative to the diabetic control group. The improvement in the PD-CSNPs group was significant (p < 0.05) compared to the PD only-treated group. Both PD and PD-CSNPs caused a marked improvement compared to the Met treatment (Fig. 3).
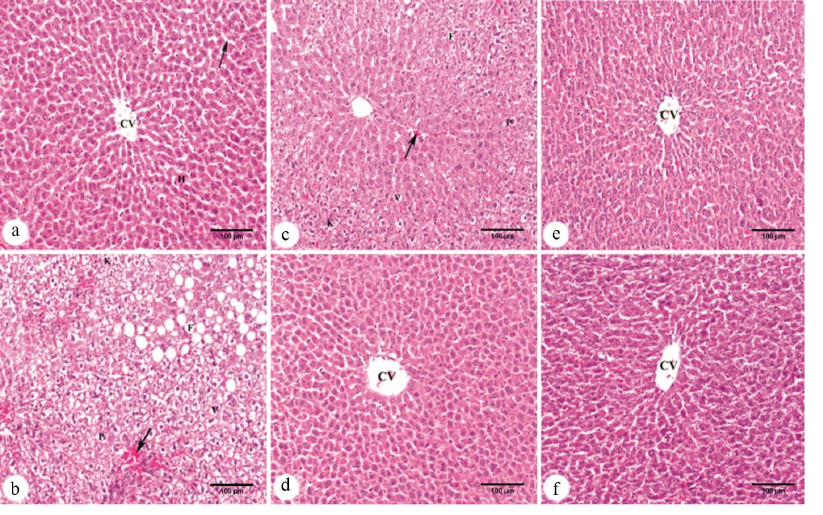
The effects of PD and PD-CSNPs on liver histology. The livers from normal control rats showed typical liver architecture with the central vein, sinusoids, and hepatocytes (Fig. 4a). The sections from the livers of control diabetic animals exhibited extensive damage, such as fatty change, dilated hyperemic sinusoids, vacuolar degeneration of hepatocytes, and appearance of hepatocytes with pyknotic and karyolytic nuclei (Fig. 4b). Diabetic animals treated with unloaded CSNPs also displayed fatty change, dilated hyperemic sinusoids, vacuolar degeneration of hepatocytes, and some hepatocytes with pyknotic and karyolytic nuclei (Fig. 4c). Treatment with PD-CSNPs caused a marked improvement in most of the liver tissue and produced a more pronounced protective effect against diabetes-induced histological abnormalities (Fig. 4d) than treatment with PD only (Fig. 4e). As expected, Met also caused improvements in the liver tissue (Fig. 4f).
Fig. 4. Micrographs of hematoxylin-eosin-stained liver sections: a) control normal rats showing normal architecture with the central vein (CV), sinusoids (arrow), and hepatocytes (H); b) control diabetic animals showing fatty change (F), dilated hyperemic sinusoids (arrow), vacuolar degeneration (V) of hepatocytes, and some hepatocytes with pyknotic (P) and karyolytic (K) nuclei; c) diabetic rats treated with CSNPs displaying fatty changes (F), dilated hyperemic sinusoids (arrow), vacuolar degeneration (V) of hepatocytes, and some hepatocytes with pyknotic (P) and karyolytic (K) nuclei; d) diabetic rats treated with PD-CSNPs showing a marked improvement in the liver tissue with the central vein (CV); e) diabetic rats treated with PD showing the central vein (CV) and moderate improvement in most hepatocytes; f) diabetic group treated with Met•HCl showing improvement in most hepatocytes and the central vein (CV). Scale bar: 100 µm.
DISCUSSION
The liver is one of the primary organs affected by the diabetes-associated chronic metabolic abnormalities that eventually cause its damage and progression of many liver disorders, such as nonalcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma [3]. PD (bioactive phytochemical) has many beneficial therapeutic properties, including antioxidant and anti-inflammatory activities [11], which make it a good candidate for alleviating liver damage in diabetic individuals. Here, we demonstrated that oral treatment with PD and PD-CSNPs for 4 weeks after diabetes induction alleviates hepatic injury in diabetic rats.
The effects of PD and PD-CSNPs as potential hypoglycemic agents in diabetic rats was similar to the effects of Met. By increasing insulin sensitivity and accelerating glucose assimilation, Met can reduce blood glucose levels [19]. The anti-hyperglycemic effect of PD and PD-CSNPs in the diabetic rats was evidenced as a decrease in the FBG levels compared to the diabetic rats. Earlier, we showed that PD and PD-CSNPs treatment caused an improvement in the insulin levels, as well as a marked reduction of glycated hemoglobin (HbA1c) content and homeostatic model assessment of insulin resistance (HOMA-IR) parameters in treated diabetic rats [15]. Compared with the control rats, diabetic rats exhibited depletion of hepatic glycogen, which was noticeably alleviated by administration of PD and PD-CSNPs. The factors leading to the depletion of hepatic glycogen in the diabetic rats include insulin deficiency, which in turn, leads to the activation of gluconeogenic and glycogenolytic pathways [20]. Glycogen is the primary intracellular storage form of glucose in the liver, and its level is a direct evidence of insulin activity, because insulin activates glycogen synthase and, consequently, stimulates glycogen deposition [21]. These results are in line with the data of Wang et al. [22], who observed elevation of hepatic glycogen in type 2 diabetic mice after PD administration.
The glucose insensitivity in type 2 diabetes is often associated with GLUT2 impairments [23]. GK is a physiological sensor of glucose in glucose-responsive tissues, including the pancreas and the liver [24]. Here, we showed that expression of GLUT2 and GK mRNAs was downregulated in the liver of diabetic rats. Treatment with PD and PD-CSNPs restored the levels of GLUT2 and GK mRNAs almost to the normal values, with a more pronounced effect observed in the PD-CSNPs group. GLUT2 is the major GLUT isoform in the liver, where it mediates the bidirectional transport of glucose, thus playing a crucial role in glucose homeostasis [25]. GLUT2 reduction leads to the loss of the glucose-induced insulin secretion by limiting the amount of glucose entering the pancreatic β cells [26], and therefore, could be one of the causes of hyperglycemia. In the liver, GLUT2 is translocated from the cytoplasm to the plasma membrane in response to the high levels of plasma glucose and acts as a major carrier of the plasma glucose into hepatocytes [27]. Beside carbohydrate metabolic disorders, the loss of GK function is associated with hyperglycemia or hypoglycemia [28], and changes in the blood glucose level are influenced by GK expression in the liver [29]. Therefore, restoration of the GLUT2 level would enhance glucose uptake and thereby, help to combat hyperglycemic conditions. Thus, the expression of GK in the liver of Zucker diabetic fatty rats decreases as the severity of the disease increases. In mice, GK overexpression in the liver improves glucose levels, while GK deficiency is associated with hyperglycemia [30].
The only pathway of glucose catabolism is glycolysis. Nutritional consumption of glucose is essential for the dietary regulation of the glycolytic pathway in the liver [31]. PK is one of the rate-limiting enzymes in the glycolytic pathway that also directly affects the rate and the direction of the entire carbohydrate metabolism [32]. PK catalysis the reaction of pyruvate and ATP synthesis via transfer of the phosphate group from phosphoenolpyruvate to ADP [33]; it accelerates glucose decomposition and promotes glycolysis. SDH is involved in both glycolysis and the tricarboxylic acid cycle (TCA) cycle [34]. Promoting the role of SDH in the electron transport chain and TCA cycle could help the liver to better utilize glucose [35]. In this study, the activities of serum PK and SDH in the diabetic rats were markedly decreased. Administration of PD and PD-CSNPs significantly increased the activities of these key carbohydrate metabolic enzymes. The elevation in the PK and SDH activities could be secondary to the ameliorative effect of PD-CSNPs on the insulin level, resulting, as mentioned above, in the promotion of glycolysis and glucose utilization for the energy generation. Our results are consistent with the data of Meghana et al. [36], who showed that administration of PD elevated SDH levels in the animal model of alcohol-induced liver injury. It was suggested that PD exhibits the anti-diabetic effects through stimulating the intracellular glucose utilization via upregulation of the GLUT2 expression, activating the pentose phosphate pathway via an increase in the SDH level, and restoring hepatic glycolysis via regulation of the key carbohydrate metabolism enzymes [37].
Protein profiles are used to diagnose the state of liver functions [38]. Our data showed a significant decrease in the serum albumin and total protein levels in the diabetic group, which might be related to an increased rate of amino acid conversion to glucose, gluconeogenesis, and conversion of glycogenic amino acids to CO2 and H2O [39]. It may also be due to the functional and structural impairments of liver cells, which are associated with the low levels of serum albumin and total protein [40]. Treating diabetic rats with PD and PD-CSNPs profoundly increased the concentrations of serum albumin and total protein, presumably, due to the hypoglycemic effect of PD. These results are consistent with the data of Ince et al. [41], who demonstrated that PD was restored in a dose-dependent manner the levels of plasma albumin and total protein altered by the cisplatin-induced toxicity in rats.
Insulin deficiency and associated hyperglycemia are caused by the dysfunction of β cells, which are considered as principal mediators in the stimulation of ROS generation accompanying liver damage in DM [42]. In our study, the liver of diabetic rats demonstrated a significant elevation of MDA content, a decrease in the GSH level, and suppression of activities of the antioxidant enzymes (SOD, GPx, CAT, and G6PD) vs. the normal control rats. Hyperglycemia-induced mitochondrial dysfunction and endoplasmic reticulum stress promote ROS accumulation, which, in turn, facilitates cell damage and contributes to the development of diabetic complications, leading to irreversible oxidative modifications [43].
NADPH is the major intracellular reducer compound. Indeed, the entire antioxidant system requires a reducer for its functioning. G6PD is the main source of NADPH, and impairments in the G6PD activity can change the NADPH levels, thus influencing the antioxidant system [44]. Accordingly, a decrease in the NADPH level resulting from the suppression of the G6PD activity, makes cells very sensitive to the oxidative damage. In line with our results, DM leads to the inhibition of G6PD activity in the experimental diabetic animals and cultured endothelial cells [45]. Diabetes patients exhibit a decreased G6PD activity in the liver [46]. Reduced G6PD activity decreases the levels of intracellular NADPH, elevates the content of intracellular ROS, and promotes oxidative stress. Furthermore, ROS can initiate LPO via oxidation of polyunsaturated fatty acid in the membranes of hepatocytes, leading to an increase in the membrane permeability followed by the cell injury [47], which can also elevate the activities of serum ALT and AST.
As predicted, PD and PD-CSNPs significantly prevented lipid oxidation by increasing the activity of the antioxidants and antioxidant enzymes (GSH, GPx, SOD, CAT, and G6PD) and by reducing the levels of MDA in the treated diabetic rats vs. the diabetic control rats. Zhang et al. [48] showed that PD attenuates hepatic oxidative stress and inhibits liver injury by decreasing the levels of MDA and increasing the activities of SOD, GSH, GPx, and CAT in the liver. The observed increase in the activity of G6PD in the rats treated with PD and PD-CSNPs explains the elevated levels of the antioxidant enzymes. NADPH, mainly produced by G6PD, is a critical CAT cofactor and glutathione reductase substrate, as it maintains CAT in the active state and ensures glutathione reduction to GSH (major free radical scavenger), respectively [49]. The antioxidant role of G6PD as a main source of NADPH has been recently well elaborated in [50].
Ozer et al. [51] reported that the activities of ALT and AST can be used as markers of liver damage. The elevated activities of these enzyme recorded in the present study were mainly due to hyperglycemia, which leads to the diabetic liver damage. We believe that oral administration of PD and PD-CSNPs provided the antioxidant protection against LPO due to the powerful activity of PD as a free radical scavenger [52] and caused a beneficial hepatoprotective effect in the case of diabetes-associated liver damage by decreasing the activities of serum ALT and AST in treated rats. These results are in agreement with the findings of Lai et al. [53], who reported similar effects for PD.
Several studies have shown that diabetes increases production of several pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, which promotes inflammation and development of hepatic steatosis, ultimately leading to the liver damage [54]. This study demonstrated that expression of IL-1β and TNF-α mRNAs was upregulated in the liver of diabetic rats, while free PD and PD-CSNPs suppressed expression of these mRNAs in the liver. PD-CSNPs produced a more pronounced anti-inflammatory effect than PD only. TNF-α is one of the major cytokines upregulated in the diabetic liver; this upregulation results in liver inflammation and apoptosis and is associated with the development of oxidative stress and hyperglycemia [55, 56]. The contribution of IL-1β in diabetes and TNF-α in alcoholic hepatitis, viral hepatitis, diabetes, and ischemia/reperfusion liver injury has also been reported [57, 58]. Due to the association between chronic inflammation and diabetic complications (including liver injury), identification of therapeutic targets that can downregulate the proinflammatory response is a promising strategy in the management of DM complications. To our knowledge, this work is the first study on the effect of PD-CSNPs as a hepatoprotective and anti-inflammatory agent alleviating diabetic liver damage. A series of studies have demonstrated that PD affects the oxidative stress and, therefore, the inflammatory response [59, 60]. The hepatoprotective effects of free PD and PD-CSNPs were manifested as stimulation of carbohydrate metabolism enzymes, inhibition of oxidative stress by upregulating antioxidant enzymes, and, consequently, modulation of pro-inflammatory cytokines.
The histological study revealed multiple histopathological changes in the liver of diabetic rats, including fatty change, dilated hyperemic sinusoids, vacuolar degeneration of hepatocytes, and some hepatocytes with pyknotic and karyolytic nuclei. These results are consistent with the previous studies that reported several histopathological abnormalities in the liver of diabetic rats [61, 62]. In line with the results of biochemical and gene expression assays, treatment of diabetic rats with PD-CSNPs and PD causes a marked improvement in most liver samples, confirming the hepatoprotective effect of these agents. However, PD-CSNPs was more efficient in liver protection compared to PD only.
Met is one of the most widely used anti-diabetic agents and is considered the first-line treatment for type 2 diabetes. The clinical advantages of Met are mainly due to its ability to specifically reduced the hepatic glucose output and to improve the peripheral insulin sensitivity; therefore, liver is the primary Met target [63]. However, Met treatment is frequently associated with several gastrointestinal side effects, such as nausea, vomiting, diarrhea, metallic taste, and abdominal discomfort. It also decreases the absorption of vitamin B12, leading to the development of anemia [64, 65]. Therefore, it is essential to develop new alternative agents of plant origin that will have no or fewer side effects for the management of diabetes and associated complications.
Considering the above-mentioned, we tested PD as a potential treatment for liver damage, as it is a safe phytochemical that has many pharmacological activities [10, 11]. Our earlier study demonstrated that CSNPs are also safe and can improve the therapeutic efficacy of PD, which might be explained by the prolonged release, improved absorption, and high bioavailability of PD in a combination with CSNPs [15]. Our study confirmed that PD-CSNPs are promising agents that have several advantages over Met, especially in the case of diabetes-associated liver damage caused by the oxidative stress. PD-CSNPs can be tailored at the molecular level to increase their efficacy and to minimize the side effects. However, before the clinical trials, the toxicokinetic and pharmacokinetic studies of the new PD-CSNPs formula are required to evaluate and to improve the balance between the efficacy and toxicity of the developed therapeutics.
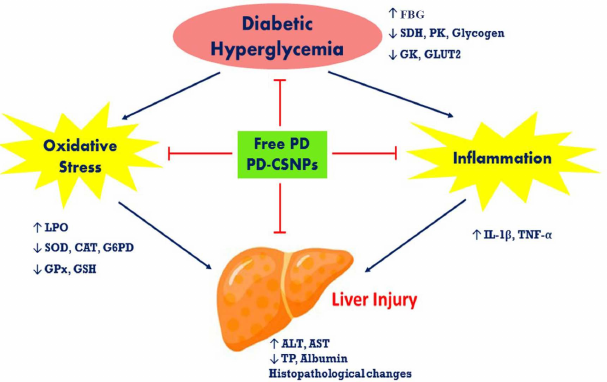
Fig. 5. The pathways of hepatoprotective action of free PD and PD-CSNPs in the liver of diabetic rats; TP, total protein.
CONCLUSION
Here, we demonstrated that PD-CSNPs are biocompatible and display a higher ameliorative effect in the diabetic liver damage compared to PD alone. We also revealed that the hepatoprotective effects of PD-CSNPs in diabetic rats may be due to the (i) anti-diabetic action via modulation of GLUT2 expression and activity of carbohydrate metabolism enzymes; (ii) modulation of the oxidative stress status due to the antioxidant properties of PD; and (iii) anti-inflammatory activity via reduction of the inflammatory cytokine content (Fig. 5).We believe that our findings will stimulate further interest in the protective role of PD-CSNPs in other liver diseases.
Ethics declarations. The authors declare no conflict of interest. All procedures performed on animals were in compliance with the ethical standards of the Institutional Animal Care and use Committee (IACUC) of the Beni-Suef University, Egypt (IACUC Permit Number: BSU-FS-2018-8).
REFERENCES
1.Cho, N., Shaw, J. E., Karuranga, S., Huang, Y., da
Rocha Fernandes, J. D., Ohlrogge, A. W., and Malanda, B. (2018) IDF
Diabetes Atlas: global estimates of diabetes prevalence for 2017 and
projections for 2045, Diabetes Res. Clin. Pract., 138,
271-281.
2.Ahlqvist, E., Van Zuydam, N. R., Groop, L. C., and
McCarthy, M. I. (2015) The genetics of diabetic complications, Nat.
Rev. Nephrol., 11, 277-287.
3.Bedi, O., Aggarwal, S., Trehanpati, N.,
Ramakrishna, G., and Krishan, P. (2019) Molecular and pathological
events involved in the pathogenesis of diabetes-associated nonalcoholic
fatty liver disease, J. Clin. Exp. Hepatol., 9,
607-618.
4.Angulo, P. (2002) Nonalcoholic fatty liver disease,
N. Engl. J. Med., 346, 1221-1231.
5.Brownlee, M. (2001) Biochemistry and molecular cell
biology of diabetic complications, Nature, 414,
813-820.
6.Lucchesi, A. N., Freitas, N. T. D., Cassettari, L.
L., Marques, S. F. G., and Spadella, C. T. (2013) Diabetes mellitus
triggers oxidative stress in the liver of alloxan-treated rats: a
mechanism for diabetic chronic liver disease, Acta Cir. Bras.,
28, 502-508.
7.Kangralkar, V. A., Patil, S. D., and Bandivadekar,
R. M. (2010) Oxidative stress and diabetes: a review, Int. J. Appl.
Pharm. Sci. Res., 1, 38-45.
8.Ingaramo, P. I., Francés, D. E., Ronco, M.
T., and Carnovale, C. E. (2013) in Hot Topics in Endocrine and
Endocrine-Related Diseases (Fedele, M., ed.), IntechOpen, London,
doi: 10.5772/53684.
9.Coman, C., Rugina, O. D., and Socaciu, C. (2012)
Plants and natural compounds with antidiabetic action, Not. Bot.
Horti. Agrobot. Cluj. Napoca., 40, 314-325.
10.Fanga, J., Luoa, L., Kea, Z., Liua, C., Yina, L.,
et al. (2019) Polydatin protects against acute cholestatic liver injury
in mice via the inhibition of oxidative stress and endoplasmic
reticulum stress, J. Funct. Foods, 55, 175-183.
11.Ji, H., Zhang, X., Du, Y., Liu, H., Li, S., and
Li, L. (2012) Polydatin modulates inflammation by decreasing
NF-κB activation and oxidative stress by increasing Gli1, Ptch1,
SOD1 expression and ameliorates blood–brain barrier permeability
for its neuroprotective effect in pMCAO rat brain, Brain Res.
Bull., 87, 50-59.
12.Cheng, W., Li, X., Zhang, C., Chen, W., Yuan, H.,
and Xu, S. (2017) Preparation and in vivo-in vitro
evaluation of polydatin-phospholipid complex with improved dissolution
and bioavailability, Int. J. Drug Dev. Res., 9,
39-43.
13.Amri, A., Chaumeil, J., Sfar, S., and Charrueau,
C. (2012) Administration of resveratrol: what formulation solutions to
bioavailability limitations? J. Control Release, 158,
182-193.
14.Nagpal, K., Singh, S., and Mishra, D. (2013)
Optimization of brain targeted chitosan nanoparticles of Rivastigmine
for improved efficacy and safety, Int. J. Biol. Macromol.,
59, 72-83.
15.Abdel-Moneim, A., El-Shahawy, A., Yousef, A. I.,
Abd El-Twab, S. M., Elden, Z. E., and Taha, M. (2020) Novel
polydatin-loaded chitosan nanoparticles for safe and efficient type 2
diabetes therapy: in silico, in vitro and in vivo
approaches, Int. J. Biol. Macromol., 154,
1496‐1504.
16.Masiello, P., Broca, C., Gross, R., Roye, M.,
Manteghetti, M., et al. (1998) Experimental NIDDM: development of a new
model in adult rats administered streptozotocin and nicotinamide,
Diabetes, 47, 224-229.
17.Seifter, S., Dayton, S., Novic, B., and
Muntwyler, E. (1950) The estimation of glycogen with the anthrone
reagent, Arch. Biochem., 25, 191-200.
18.Bancroft, J. D., and Gamble, M. (2002) In
Theory and Practice of Histological Techniques, 5th Ed.,
Churchill Livingstone, pp. 172-175.
19.Xu, C., Liu, W., Zhang, D., Cao, X., Shi, H., and
Li, X. (2018) Interactions between dietary carbohydrate and metformin:
implications on energy sensing, insulin signaling pathway, glycolipid
metabolism and glucose tolerance in blunt snout bream Megalobrama
amblycephala, Aquaculture, 483, 183-195.
20.Pari, L., and Murugan, P. (2005) Effect of
tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key
enzymes in streptozotocin induced diabetic rats, J. Basic Clin.
Physiol. Pharmacol., 16, 257‐274.
21.Vats, V., Yadav, S., and Grover, J. (2004)
Ethanolic extract of Ocimum sanctum leaves partially attenuates
streptozotocin-induced alterations in glycogen content and carbohydrate
metabolism in rats, J. Ethnopharmacol., 90, 155-160.
22.Wang, Y., Ye, J., Li, J., Chen, C., Huang, J.,
Liu, P., and Huang, H. (2016) Polydatin ameliorates lipid and glucose
metabolism in type 2 diabetes mellitus by downregulating proprotein
convertase subtilisin/kexin type 9 (PCSK9), Cardiovasc.
Diabetol., 15, 19, doi: 10.1186/s12933-015-0325-x.
23.Thorens, B., Wu, Y. J., Leahy, J. L., and Weir,
G. C. (1992) The loss of GLUT2 expression by glucose unresponsive beta
cells of db/db mice is reversible and is induced by the diabetic
environment, J. Clin. Invest., 90, 77-80.
24.Liu, D., Regenstein, J. M., Diao, Y., Qiu, J.,
Zhang, H., Li, J., Zhao, H., and Wang, Z. (2019) Antidiabetic effects
of water-soluble Korean pine nut protein on type 2 diabetic mice,
Biomed. Pharmacother., 117, 108989, doi:
10.1016/j.biopha.2019.108989.
25.Sole, S. S., and Srinivasan, B. P. (2012) Aqueous
extract of tamarind seeds selectively increases glucose transporter-2,
glucose transporter-4, and islets’ intracellular calcium levels
and stimulates β-cell proliferation resulting in improved glucose
homeostasis in rats with streptozotocin-induced diabetes mellitus,
Nutr. Res., 32, 626-636.
26.Weir, G. C. (1993) The relationship of diabetes,
loss glucose-induced insulin secretion, and GLUT2, J. Diabetes
Complications, 7, 124-129.
27.Zhao, F. Q., and Keating, A. F. (2007) Expression
and regulation of glucose transporter in the bovine mammary gland,
J. Dairy Sci., 90, E76-E86.
28.Gloyn, A. L., Odili, S., Zelent, D., Buettger,
C., Castleden, H. A. J., et al. (2005) Insights into the structure and
regulation of glucokinase from a novel mutation (V62M), which causes
maturity-onset diabetes of the young, J. Biol. Chem.,
280, 14105‐14113.
29.Zhang, Y., Wei, Z., Liu, G., Deng, K., Yang, M.,
et al. (2019) Synergistic effects of dietary carbohydrate and taurine
on growth performance, digestive enzyme activities and glucose
metabolism in juvenile turbot Scophthalmus maximus L.,
Aquaculture, 499, 32-41.
30.Postic, C., Shiota, M., Niswender, K. D., Jetton,
T. L., Chen, Y., et al. (1999) Dual roles for glucokinase in glucose
homeostasis as determined by liver and pancreatic beta cell-specific
gene knock-outs using Cre recombinase, J. Biol. Chem.,
274, 305‐315.
31.Li, R., Liu, H., Dong, X., Chi, S., Yang, Q.,
Zhang, S., and Tan, B. (2018) Molecular characterization and expression
analysis of glucose transporter1 and hepatic glycolytic enzymes
activities from herbivorous fish Ctenopharyngodon idellus in respond to
a glucose load after the adaptation to dietary carbohydrate levels,
Aquaculture, 792, 290-299.
32.Kayne, F. J (1973) Pyruvate kinase, in The
Enzymes, 3rd Edn., Vol. VIII (Boyer, P. D., ed.) Academic Press,
New York, pp. 353-382.
33.Gupta, V., and Bamezai, R. N., (2010) Human
pyruvate kinase M2: a multifunctional protein, Protein Sci.,
19, 2031-2044.
34.Oyedotun, K. S., and Lemire, B. D. (2004) The
quaternary structure of the saccharomyces cerevisiae succinate
dehydrogenase, J. Biol. Chem., 279, 9424-9431.
35.Choudhury, H., Pandey, M., Hua, C. K., Mun, C.
S., Jing, J. K., et al. (2017) An update on natural compounds in the
remedy of diabetes mellitus: a systematic review, J. Tradit.
Complement. Med., 8, 361-376.
36.Koneru, M., Sahu, B. D., Gudem, S., Kuncha, M.,
Ravuri, H. G., Kumar, J. M., Kilari, E. K., and Sistla, R. (2017)
Polydatin alleviates alcohol-induced acute liver injury in mice:
Relevance of matrix metalloproteinases (MMPs) and hepatic antioxidants,
Phytomedicine, 27, 23‐32.
37.Enes, P., Panserat, S., Kaushik, S., and
Oliva-Teles, A. (2009) Nutritional regulation of hepatic glucose
metabolism in fish, Fish Physiol. Biochem., 35,
519-539.
38.Soji-Omoniwa, O., Muhammad, N. O., Usman, L. A.,
and Omoniwa, B. P. (2014) Effect of leaf essential oil of Citrus
sinensis at different harvest time on some liver and kidney function
indices of diabetic rats, Int. J. Biol. Vet. Agric. Food Eng.,
8, 484-488.
39.Abdel-Moneim, A., Abd El-Twab, S. M., Ashour, M.
B., and Yousef, A. I. (2016) Hepato-renal protective effects of gallic
acid and p-coumaric acid in nicotinamide/streptozotocin-induced
diabetic rats, Int. J. Bioassays, 5.6, 4641-4649.
40.Korish, A. A., and Arafah, M. M. (2013) Camel
milk ameliorates steatohepatitis, insulin resistance and lipid
peroxidation in experimental non-alcoholic fatty liver disease, BMC
Complement Altern. Med., 13, 264, doi:
10.1186/1472-6882-13-264.
41.Ince, S., Arslan Acaroz, D., Neuwirth, O.,
Demirel, H. H., et al. (2014) Protective effect of polydatin, a natural
precursor of resveratrol, against cisplatin-induced toxicity in rats,
Food Chem. Toxicol., 72, 147‐153.
42.Schmatz, R., Perreira, L. B., Stefanello, N.,
Mazzanti, C., Spanevello, R., et al. (2012) Effects of resveratrol on
biomarkers of oxidative stress and on the activity of delta
aminolevulinic acid dehydratase in liver and kidney of
streptozotocin-induced diabetic rats, Biochimie, 94,
374‐383.
42.Fiorentino, T. V., Prioletta, A., Zuo, P., and
Folli, F. (2013) Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases, Curr. Pharm.
Des., 19, 5695‐5703.
44.Xu, Y., Osborne, B. W., and Stanton, R. C. (2005)
Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via
activation of PKA, which contributes to oxidative stress in rat kidney
cortex, Am. J. Physiol. Renal. Physiol., 289,
F1040‐F1047.
45.Ulusu, N. N., Sahilli, M., Avci, A., Canbolat,
O., Ozansoy, G., et al. (2003) Pentose phosphate pathway,
glutathione-dependent enzymes and antioxidant defense during oxidative
stress in diabetic rodent brain and peripheral organs: effects of
stobadine and vitamin E, Neurochem. Res., 28,
815‐823.
46.Cedola, N., Cabarrou, A., Auciello, N., Doria,
I., Ponce, D. L., and Baylon, N. (1974) The liver in human diabetes.
Concentration of some induced enzymes, Acta Diabetologica,
12, 263-271.
47.Hosni, A. A., Abdel-Moneim, A. A., Abdel-Reheim,
E. S., Mohamed, S. M., and Helmy, H. (2017) Cinnamaldehyde potentially
attenuates gestational hyperglycemia in rats through modulation of
PPARγ, proinflammatory cytokines and oxidative stress, Biomed.
Pharmacother., 88, 52‐60.
48.Zhang, H., Yu, C. H., Jiang, Y. P., Peng, C., He,
K., Tang, J. Y., and Xin, H. L. (2012) Protective effects of polydatin
from Polygonum cuspidatum against carbon tetrachloride-induced liver
injury in mice, PLoS One, 7, e46574, doi:
10.1371/journal.pone.0046574.
49.Kirkman, H. N., Rolfo, M., Ferraris, A. M., and
Gaetani, G. F. (1999) Mechanisms of protection of catalase by NADPH.
Kinetics and stoichiometry, J. Biol. Chem., 274,
13908‐13914.
50.Leopold, J. A., Zhang, Y. Y., Scribner, A. W.,
Stanton, R. C., and Loscalzo, J. (2003) Glucose-6-phosphate
dehydrogenase overexpression decreases endothelial cell oxidant stress
and increases bioavailable nitric oxide, Arterioscler. Thromb. Vasc.
Biol., 23, 411‐417.
51.Ozer, J., Ratner, M., Shaw, M., Bailey, W., and
Schomaker, S. (2008) The current state of serum biomarkers of
hepatotoxicity, Toxicology, 245, 194-205.
52.Huang, Q. H., Xu, L. Q., Liu, Y. H., Wu, J. Z.,
Wu, X., et al. (2017) Polydatin protects rat liver against
ethanol-induced injury: involvement of CYP2E1/ROS/Nrf2 and
TLR4/NF-κB p65 pathway, Evid. Based. Complement Alternat.
Med., 2017, 7953850, doi: 10.1155/2017/7953850.
53.Lai Xue, L., Wub, K., Qiu, H., Huang, B., Chen,
R., Xie, W., and Jiang, Q. (2017) Polydatin exhibits the
hepatoprotective effects through PPAR-a/-b signaling pathway in
streptozocin-induced diabetic mice, J. Funct. Foods, 36,
341-347.
54.Perumpail, R. B. (2015) Pathogenesis of
hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease:
potential mechanistic pathways, World J. Gastroenterol.,
7, 2384-2388.
55.Ingaramo, P. I., Ronco, M. T., Francés, D.
E., Monti, J. A., Pisani, G. B., et al. (2011) Tumor necrosis factor
alpha pathways develops liver apoptosis in type 1 diabetes mellitus,
Mol. Immunol., 48, 1397‐1407.
56.Xiao, Y., Chen, L., Fan, Y., Yan, P., Li, S., and
Zhou, X. (2019) The effect of boletus polysaccharides on diabetic
hepatopathy in rats, Chem. Biol. Interact., 308,
61‐69.
57.Ehses, J., Lacraz, G., Giroix, M., Schmidlin, F.,
Coulaud, J., et al. (2009) IL-1 antagonism reduces hyperglycemia and
tissue inflammation in the type 2 diabetic GK rat, Proc. Natl. Acad.
Sci. USA, 106, 13998-14003.
58.Ding, W., and Yin, X. (2004) Dissection of the
multiple mechanisms of TNF‐α‐induced apoptosis in
liver injury, J. Cell Mol. Med., 8, 445-454.
59.Du, Q. H., Peng, C., and Zhang, H. (2013)
Polydatin: a review of pharmacology and pharmacokinetics, Pharm.
Biol., 51, 1347-1354.
60.Li, T., Cai, S., Zeng, Z., Zhang, J., Gao, Y.,
Wang, X., and Chen, Z. (2014) Protective effect of polydatin against
burn-induced lung injury in rats, Respir. Care, 59,
1412-1421.
61.Teoh, S. L., Latiff, A. A., and Das, S. (2009) A
histological study of the structural changes in the liver of
streptozotocin-induced diabetic rats treated with or without Momordica
charantia (bitter gourd), Clin Ter., 160, 283-286.
62.Song, D., Yin, L., Wang, C., and Wen, X. (2020)
Zhenqing recipe attenuates non-alcoholic fatty liver disease by
regulating the SIK1/CRTC2 signaling in experimental diabetic rats,
BMC Complement. Altern. Med., 20, 27, doi:
10.1186/s12906-019-2811-2.
63.Song, R. (2016) Mechanism of metformin: a tale of
two sites, Diabetes Care, 39, 187-189.
64.Nathan, D. M., Buse, J. B., Davidson, M. B.,
Ferrannini, E., Holman, R. R., Sherwin, R., Zinman, B., American
Diabetes Association, and European Association for Study of Diabetes
(2009) Medical management of hyperglycemia in type 2 diabetes: a
consensus algorithm for the initiation and adjustment of therapy
– a consensus statement of the American Diabetes Association and
the European Association for the Study of Diabetes, Diabetes
Care, 32, 193-203.
65.Abdel-Moneim, A, Abdel-Reheim, E. S., Semmler,
M., and Addaleel, W. (2019) The impact of glycemic status and metformin
administration on red blood cell indices and oxidative stress in type 2
diabetic patients, Malays J. Med. Sci., 26, 47-60.