Antioxidant Properties of Galanin and Its N-Terminal Fragments in in vitro and in vivo Oxidative Stress Modeling
Oleg I. Pisarenko1,a*, Irina M. Studneva1, Larisa I. Serebryakova1, Alexandr A. Timoshin1, Galina G. Konovalova1, Vadim Z. Lankin1, Alla K. Tihaze1, Oksana M. Veselova1, Igor V. Dobrokhotov1, Roman O. Lyubimov1, Mariya V. Sidorova1, Marina E. Palkeeva1, and Alexandr S. Molokoedov1
1National Medical Research Center for Cardiology, Ministry of Health of the Russian Federation, 121552 Moscow, Russia* To whom correspondence should be addressed.
Received December 21, 2020; Revised February 4, 2021; Accepted February 8, 2021
Antioxidant properties of rat galanin GWTLNSAGYLLGPHAIDNHRSFSDKHGLT-NH2 (Gal), N-terminal fragment of galanin (2-15 aa) WTLNSAGYLLGPHA (G1), and its modified analogue WTLNSAGYLLGPβAH (G2) were studied in vivo in the rat model of regional myocardial ischemia and reperfusion and in vitro in the process of Cu2+-induced free radical oxidation of human blood plasma low-density lipoproteins. Intravenous administration of G1, G2, and Gal to rats after ischemia induction reduced the infarction size and activities of the necrosis markers, creatine kinase-MB and lactate dehydrogenase, in blood plasma at the end of reperfusion. G1, G2, and Gal reduced formation of the spin adducts of hydroxyl radicals in the interstitium of the area at risk during reperfusion, moreover, G2 and Gal also reduced formation of the secondary products of lipid peroxidation in the reperfused myocardium. It was shown in the in vivo experiments and in the in vitro model system that the ability of galanin peptides to reduce formation of ROS and attenuate lipid peroxidation during myocardial reperfusion injury was not associated directly with their effects on activities of the antioxidant enzymes of the heart: Cu,Zn-superoxide dismutase, catalase, and glutathione peroxidase. The peptides G1, G2, and Gal at concentrations of 0.01 and 0.1 mM inhibited Cu2+-induced free radical oxidation of human low-density lipoproteins in vitro. The results of oxidative stress modeling demonstrated that the natural and synthetic agonists of galanin receptors reduced formation of the short-lived ROS in the reperfused myocardium, as well as of lipid radicals in blood plasma. Thus, galanin receptors could be a promising therapeutic target for cardiovascular diseases.
KEY WORDS: galanin, heart, ischemia and reperfusion, necrosis, lipid peroxidation, antioxidant enzymes, cardiomyocyte membrane damageDOI: 10.1134/S0006297921040106
Abbreviations: AAR, area at risk; BHT, butylated hydroxytoluene; CAT, catalase; CK-MB, creatine kinase-MB; Cu,Zn-SOD, Cu,Zn-superoxide dismutase; DMPO, 5,5-dimethyl-1-pyrrolin-N-oxide; DMSO, dimethyl sulfoxide; GSH-Px, glutathione peroxidase; I/R, ischemia/reperfusion; LDH, lactate dehydrogenase; LDL, low density lipoproteins; LPO, lipid peroxidation; LV, left ventricle; MI, myocardial infarction; ROS, reactive oxygen species.
INTRODUCTION
A neuropeptide galanin comprising 29 amino acid (aa) residues (30 in humans) is involved in vitally important processes: memory, food consumption, falling asleep, production of some hormones; on the cellular level it participates in maintaining ionic homeostasis and osmosis. This peptide also plays a role in the development of alcohol dependence and neuropathic pain. In peripheral organs, including heart, the action of galanin is mediated not only through the neuronal mechanisms, but also through activation of the transmembrane receptors GalR1, GalR2, and GalR3 [1]. Binding with the receptors is controlled by the N-terminal fragment of the peptide with the first 15 aa conserved among the majority of species. The role of galanin receptors in cardiovascular system regulation in normal and pathological conditions is poorly understood. The recent studies have shown that triggering the signaling pathway through the GalR2 receptor by the N-terminal fragments of galanin H-Trp-Thr-Leu-Asn-Ser-Ala-Gly-Tyr-Leu-Leu-OH (2-11) and H-Trp-Thr-Leu-Asn-Ser-Ala-Gly-Tyr-Leu-Leu-Gly-Pro-His-Ala-OH (2-15) (G1) inhibited apoptosis during hypoxia/reoxygenation of the isolated rat cardiomyocytes and cardiomyoblasts of the cell line H9c2 due to decrease in the production of superoxide anion-radicals and hydrogen peroxide in mitochondria [2, 3]. Both peptides promote metabolic and functional recovery of the rat heart after the damage induced by ischemia/reperfusion (I/R) ex vivo and in vivo. Taking into consideration the abovementioned properties of the peptides it seems promising to use pharmacological agonists of the galanin receptors to reduce damage to the ischemic heart. To optimize physicochemical properties of the galanin fragments, a number of modified analogs of the G1 peptide that retain pharmacophoric aa responsible for binding with the GalR2 receptor were synthesized. General formula of these peptides is as follows: Trp-Thr-Leu-Asn-Ser-Ala-Gly-Tyr-Leu-X-Gly-Pro-Y, where X = Leu, Y = His-Ala-NH2; or X = Nle, Y = His-Arg-OH; or X = Leu, Y = bAla-His-OH (G2). The study of these peptides in the models of myocardial I/R injury revealed their cardiotropic properties [4]. It was demonstrated in the present work that the chimeric molecule of G2 containing the galanin sequence (2-13) supplemented by the natural dipeptide carnosine H-Trp-Thr-Leu-Asn-Ser-Ala-Gly-Tyr-Leu-Leu-Gly-Pro-bAla-His-OH was the most effective [5]. Administration of the peptide G2 was shown to reduce heart dysfunction in rats with doxorubicin-induced cardiomyopathy [6]. The protective effect was accompanied by the decrease in the lipid peroxidation (LPO) as a result of the increase in activities of Cu,Zn-superoxide dismutase (Cu,Zn-SOD) and glutathione peroxidase (GSH-Px) in the damaged myocardium, and also by the improvement of the energy supply to cardiomyocytes. The present work continues the study on antioxidant properties of the galanin receptor ligands. The objectives of this work were as follows: (i) to study effects of the synthetic peptide G2, the natural fragment G1 of galanin (2-15), and of the full-size rat galanin (1-29) (Gal) on activities of Cu,Zn-SOD, GSH-Px, and catalase (CAT) as well as on production of reactive oxygen species (ROS) and LPO products in the rat ischemic heart in vivo; (ii) to study effects of the abovementioned peptides on the activities of the enzymes Cu,Zn-SOD, GSH-Px, and CAT in the model systems in vitro; (iii) to assess effects of the peptides on Cu2+-induced free radical oxidation of the low density lipoproteins (LDL) in vitro.
MATERIALS AND METHODS
Galanin peptides. Gal and its N-terminal fragments G1 and G2 produced using a stepwise solid-phase synthesis with Fmoc-methodology in the laboratory of peptide synthesis of the National Medical Research Center for Cardiology, Ministry of Health of Russian Federation were used in this work [5, 7]. Gal and its fragments were purified using reverse phase high performance liquid chromatography, and their structure was characterized with 1H-NMR-spectroscropy and MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization-Time of Flight) mass-spectrometry. Characteristics of the peptides are presented in Table 1.
Table 1. Characteristics of galanin
peptides

Note. Amino acid substitutions are shown in bold.
Animals. Effect of the peptides on the heart during ischemia and reperfusion was studied in male Wistar rats (body weight 300-350 g). The animals were kept in a vivarium under conditions of a natural light-dark cycle, and water and feed access ad libitum. All international, national, and/or institutional guidelines for the care of laboratory animals and their use in experiments were followed.
Modeling of regional ischemia and reperfusion in rats in vivo. Wistar rats were anesthetized with 20% urethane (1200 mg/kg body weight intraperitoneally) and an artificial lung ventilation with the room air was provided under conditions of thoracotomy using a KTR-5 apparatus (Hugo Sacks Electronik, Germany). The jugular vein was catheterized to stain the heart with 1% Evans solution at the end of procedure, the carotid vein was catheterized to monitor blood pressure. Systolic arterial pressure and heart rate were recorded with a polygraph Biograph-4 (Saint-Petersburg State University of Aerospace Instrumentation, Russia) by connecting the arterial catheter to a tensometric sensor. Digitizing of the signal was performed with an analog-to-digital converter USB-6210 and the LabView 7 program (National Instruments, USA) [5]. After the animal was prepared for experiment, there was a 30-min period for stabilization of hemodynamic parameters (later “the initial state”), and next the anterior descending coronary artery (ADCA) was occluded for 40 min followed by 60-min period of reperfusion. In the experimental series after the period of regional ischemia bolus injection of the peptides Gal, G1 and G2 in the doses of 0.25, 0.5, 1.0, 2.0, or 3.0 mg/kg body weight was performed at the onset of reperfusion; in the control series of experiments a similar volume (0.5 ml) of saline was injected. In a separate series of experiments the effect of the peptide solvent (0.2% dimethyl sulfoxide, DMSO) on the myocardial infarction (MI) size was examined. At the end of procedure, the ADCA was re-occluded and bolus injection of 2% Evans solution (2 ml) into the jugular vein was performed for identification of the area at risk (AAR) and the intact area of myocardium. Next the heart was excised and left ventricle (LV) was isolated for determination of the MI size.
Assessment of the necrotic death of cardiomyocytes in the area at risk. The degree of necrosis of cardiomyocytes in the AAR was determined by the MI area using the computer planimetry with the ImageJ program (NIH, USA). The frozen LV was cut perpendicularly to the long axis of the heart to obtain 4-5 slices with the thickness of ≈1.5-2.0 mm, which were incubated for 10 min in a 1% solution of 2,3,5-triphenyltetrazolium chloride in 0.1 M potassium-phosphate buffer (pH 7.4, at 37°C) and fixed in 10% formalin. The resulting specimens were scanned for determination of the MI area and the AAR in the acquired images. The slices were weighed to determine the LV mass. In each group the ratios of the “AAR/left ventricle mass” (AAR/LV) and “myocardial infarction/AAR” (MI/AAR) were calculated and expressed in percent [8].
Assessment of the cell membrane damage. Damage to the membranes of cardiomyocytes was assessed from the increase in activities of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) in the blood plasma. About 0.5 ml of blood was collected into heparin-coated tubes from the venous catheter of rats in the initial state (before the ADCA occlusion) and after 1 h of reperfusion. The enzyme activities in the blood plasma were determined with an UV-1800 spectrophotometer (Shimadzu, Japan) at λ = 340 nm using reagent kits from BioSystems SA (Spain).
Determination of activities of antioxidant enzymes and levels of lipid peroxidation products in the AAR. Tissue samples from the AAR frozen in liquid nitrogen were homogenized in a 50 mM sodium-phosphate buffer (pH 7.4; w/v 1 : 10) using an Ultra-Turrax T18 homogenizer (IKA Werke, Germany) and centrifuged in a Sigma-Aldrich 3-16KL centrifuge (Germany) at 1,000g and 4°C for 10 min. LPO secondary products [2-thiobarbituric acid reactive substances (TBARS)] and activities of the antioxidant enzymes (Cu,Zn-SOD, GSH-Px, and CAT) were determined in the supernatant. Cu,Zn-SOD activity was determined from inhibition of p-Nitrotetrazolium Blue reduction by the superoxide radical generated in the system of xanthine-xanthine oxidase. Kinetics of formazan generation was recorded with an UV-2600 spectrophotometer (Shimadzu) at λ = 560 nm. The amount of Cu,Zn-SOD required for 50% suppression of p-Nitrotetrazolium Blue reduction was taken as the enzyme activity unit; the results were expressed in activity units/mg protein [9]. The CAT activity was determined by the rate of H2O2 consumption at 20°C during 1 min. The measurements were performed at 240 nm with an UV-2600 spectrophotometer. Activity was calculated using hydrogen peroxide molar extinction coefficient ε = 43.6 M—1·cm—1. Activity unit was defined as the amount of enzyme required for utilization of 1 µmol H2O2 per minute; the results were expressed also in activity units/mg protein [10]. Activity of GSH-Px was determined in the conjugated system of glutathione-glutathione reductase by oxidation of NADPH, using tert-butyl peroxide as a substrate. The rate of NADPH oxidation (λ = 340 nm) was determined in a thermostated 9-channel cuvette at 30°C with a FP-900 chemical analyzer (Labsystems Oy, Finland). The amount of enzyme required for oxidation of 1 µmol of reduced glutathione under assay conditions was taken as an activity unit; the results were expressed in activity units/mg protein [11]. Proteins in the supernatant of the cardiac muscle homogenate were precipitated with 10% trichloroacetic acid (1 : 1) and level of the secondary products of lipid peroxidation were determined using the reaction with 2-thiobarbituric acid by analyzing quantity of the produced trimethine complex at λ = 532 nm using an UV-2600 spectrophotometer [12].
Assessment of the effect of peptides on activities of the antioxidant enzymes. Commercial preparations of Cu,Zn-SOD and GSH-Px from bovine erythrocytes and of CAT from bovine liver (Sigma-Aldrich, USA) were dissolved in 50 mM phosphate buffer (pH 7.4) to provide concentration of 250 µg/ml. The enzyme activities were 145.3 ± 3.19, 462.8 ± 43.07, and 12.73 ± 0.45 U/ml, respectively. Next G1, G2, and Gal peptides dissolved in 50 mM phosphate buffer (pH 7.4) were added to the enzyme-containing solutions at final concentrations of 0.01 and 0.1 mM and the resulting mixtures were incubated at 4°C for 24 h. Activities of Cu,Zn-SOD, CAT, and GSH-Px were determined on completion of incubation as described in [9-11].
Study of the effect of peptides on free radical oxidation of human LDL. For preparative isolation of low-density lipoproteins (LDL), donor blood plasma containing EDTA (1 mg/ml) was centrifuged twice in the density gradient 1.019-1.063 g/cm3 of NaBr for 2 h at 42,000 rpm in an angle rotor 50 Ti at 4°C in a refrigerated ultracentrifuge Optima XPN-80 (Beckman Coulter, USA) as described in [13]. The resulting LDL were dialyzed against an isotonic (0.154 M NaCl) 50 mM K,Na-phosphate buffer (pH 7.4) at 4°C for 16 h. The short time of centrifugation makes it possible to prevent oxidation of the native LDL in the course of isolation. Electrophoresis of LDL specimens prepared as described above shown no significant contamination with other lipoprotein fractions or plasma proteins.
Protein level in the dialyzed LDL specimens was determined with the Lowry assay. Next the protein samples were diluted to concentration 50 µg/ml with a solution containing 0.154 M NaCl in 50 mM K,Na-phosphate buffer (pH 7.4) and galanin peptides were added at concentrations of 0.01 and 0.1 mM. Oxidation of LDL was induced at 37°C by introduction of 30 µM CuSO4·5H2O, and next accumulation of lipohydroxyperoxides was determined at fixed time intervals at λ = 233 nm with an UV-2600 spectrophotometer. The degree of inhibition of free radical oxidation of LDL was determined from the duration of induction period (lag-phase) of oxidation (τ), which was defined by the time optical density at λ = 233 nm on the kinetic curves of LDL oxidation reached the level of 0.15 (ΔD233 = 0.15) [13]. Ethanol solution of synthetic phenolic antioxidant (0.01 mM butylated hydroxytoluene (BHT), final ethanol concentration was 0.25%) was added to the reference sample. It was shown in the preliminary experiments that introduction of 0.25% ethanol into the sample did not affect duration of the lag-phase in the process of LDL oxidation.
Monitoring of ROS production in the AAR of rat heart with a spin trap. The level of short-lived oxygen radicals in the LV area at risk was monitored by microdialysis and spin trapping with 5,5-dimethyl-1-pyrrolin N-oxide (DMPO). This compound can effectively interact with both superoxide and hydroxyl radicals producing relatively stable spin adducts that can be detected using electron paramagnetic resonance (EPR) technique [14]. A microdialysis fiber (external diameter was 0.25 mm, permeable for substances with molecular mass <5 kDa) was implanted into the area of regional ischemia, and it was perfused with a Carnegie Medicine Micro-Injection Pump, Model CMA 100 at the rate of 3 µl/min with Ringer solution (pH 7.4 at 37°C) containing 100 mM DMPO. The leaking dialysate was collected as the successive 20-min fractions into plastic tubes cooled to 0°C. The dialyzed specimens were frozen and kept in liquid nitrogen. EPR spectra of the dialysate specimens containing the DMPO spin trap were recorded with a Varian E-109 E X-band electron spin resonance spectrometer (USA) at room temperature. Amplitude of high frequency magnetic field modulation was 0.1 mTl at frequency of 100 kHz. Microwave frequency of the spectrometer was 9.14 GHz and its power was set at 10 mW. Magnetic field was scanned with a center at g = 2.00 during signal recording.
Statistical processing of data. The data were processed with a program package SigmaPlot 11.2 (SysStat, USA). The results are presented as mean ± standard error of the mean (M ± SEM). The Student’s t-test with Bonferroni correction was used for comparison of several groups with the control. Differences were considered significant at p < 0.05.
RESULTS
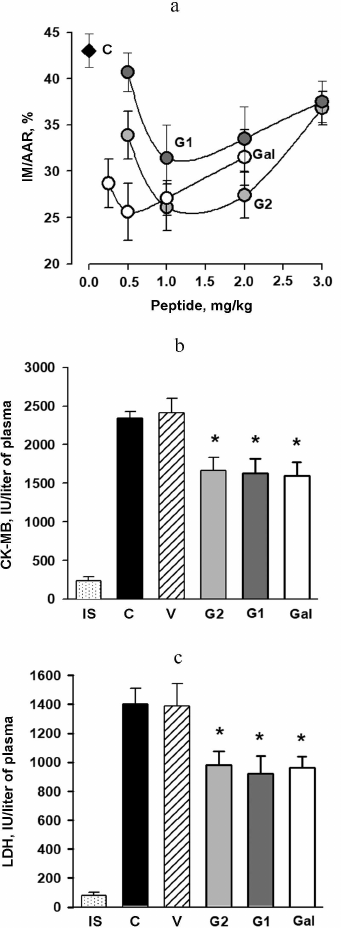
Effects of the peptides on myocardial I/R injury in rats in vivo. Histochemical analysis of the LV slices after reperfusion did not reveal significant differences in the AAR size in the control, galanin peptides, and 0.2% DMSO-treated rats. The AAR/LV ratio in the studied groups was, on average, 40.5 ± 1.3% indicating the same degree of I/R injury in all animals. The MI size in the control group expressed as MI/AAR ratio in percent was 43.0 ± 2.0% (Fig. 1a). Injection of 0.2% DMSO did not affect the MI size: in this case the MI/AAR ratio was 41.6 ± 3.1%. However, intravenous injection of any dose of the peptides under study led to the reduction of the MI size. The optimal dose for Gal was 0.5 mg/kg, for G1 and G2 it was 1.0 mg/kg. Using of these doses of Gal and G2 caused, on average, 40% decrease in the MI size, whereas G1 caused a 27% decrease as compared to the control (p < 0.001). Development of MI in the control group at the end of reperfusion was accompanied by the significant increase of activities of CK-MB and LDH in the blood plasma as compared to the initial state (Fig. 1, b and c). Injection of 0.2% DMSO did not affect activities of both enzymes as compared to the control. Injections of the optimal doses of Gal, G1, and G2 caused, on average, a 30% reduction of the LDH and CK-MB activities at the end of reperfusion, as compared to the control (p < 0.01). These data indicate decrease in cardiomyocyte necrosis in the AAR under the effect of galanin and its N-terminal fragments. Further in the study the abovementioned optimal concentrations of the peptides were used in the experiments.
Fig. 1. Effects of intravenous injections of galanin peptides on the parameters of myocardial I/R injury in rats in vivo. a) Dose-dependent effect of the peptides Gal, G1, and G2 on the myocardial infarction size (MI/AAR, %): C is control, MI is myocardial infarction, AAR is area at risk. Effect of optimal doses of the peptides on activities of CK-MB (b) and LDH (c) in the blood plasma of rats at the end of reperfusion: IS, initial state; C, control (injection of saline); V, the vehicle; 0.2% DMSO, peptides: G1 (1 mg/kg), G2 (1 mg/kg), Gal (0.5 mg/kg). The data are presented as M ± SEM for each group of eight animals. * A significant difference from the C and V (p < 0.01).
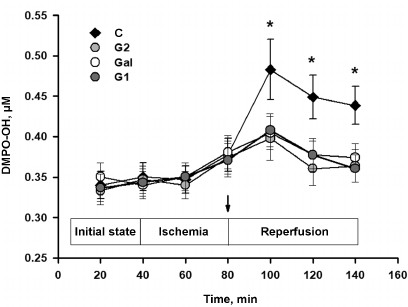
Effect of the peptides on ROS production in the AAR of rat heart. The recorded EPR spectra of the dialysate specimens comprised four narrow equidistant lines with the amplitude ratio 1 : 2 : 2 : 1 characteristic for the paramagnetic adduct DMPO-OH produced as a result of DMPO interaction with hydroxyl radical [14]. Figure 2 presents changes in the adduct DMPO-OH content in the dialysate specimens in the course of experiment. One can see that the DMPO-OH content in the dialysate increased in the control after 40-min occlusion of the coronary artery, which indicated a similar increase in the ROS level in the interstitium of the AAR. This could be associated with the significant increase in the ROS generation in the respiratory chain of mitochondria during the blood flow recovery in the AAR. Injections of the peptides G1, G2, or Gal before the reperfusion significantly decreased the content of DMPO-OH as compared to the control (p < 0.01). These data indicate the decrease in the ROS production in the reperfused myocardium under the effect of galanin peptides.
Fig. 2. Effect of the galanin peptides on concentration of the DMPO-OH adduct in the interstitium of the AAR of the rat heart. C, control (injection of saline), the peptides: G1 (1 mg/kg), G2 (1 mg/kg), Gal (0.5 mg/kg). The data are presented as M ± SEM for each group of five animals. * Significant difference from G1, G2, and Gal (p < 0.01); the arrow indicates the time point of the peptide injection.
Effect of the peptides on activities of the antioxidant enzymes and contents of LPO products in the AAR of rat heart. The activities of Cu,Zn-SOD, CAT, and GSH-Px in the AAR in the control (after the regional ischemia and reperfusion of the heart without injection of the peptides) were not different from their values in the initial state (Table 2). The Cu,Zn-SOD activity in the AAR did not change under the effect of G2 and Gal at the end of reperfusion, whereas injection of G1 caused its decrease. The CAT activity was not changed significantly under the effect of the galanin peptides. The GSH-Px activity in the AAR increased significantly as compared to the control after injections of the G2 and Gal peptides, whereas injection of G1 did not produce any effects. The TBARS content in the AAR increased 2-fold under the effect of regional ischemia and reperfusion in the control as compared to the initial value. The injections of G2 and Gal noticeably decreased accumulation of TBARS in the AAR at the end of reperfusion, whereas G1 had no effect on this parameter. Thus, the peptides G2 and Gal caused decrease in the formation of LPO products in the reperfused myocardium, despite the absence of the effect on activities of Cu,Zn-SOD and CAT and slight increase in the GSH-Px activity. The nearly 2-fold decrease in the Cu,Zn-SOD activity caused by the injection of G1 had no effect on the LPO intensity in the AAR as compared to the control. These data indicate that the changes observed in the LPO in the reperfused heart region under the effect of the peptides are not directly associated with the activities of the key antioxidant enzymes.
Table 2. Activities of the antioxidant
enzymes and the content of TBARS (LPO products) in the rat heart
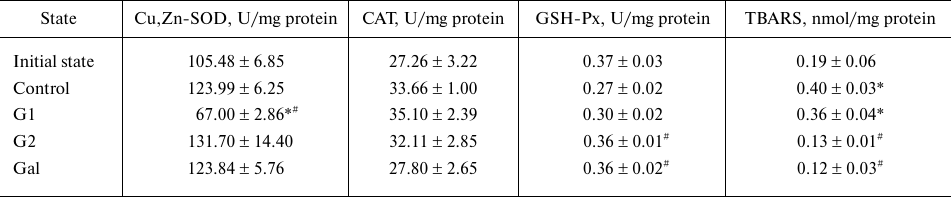
Note. Data are presented for the series of eight experiments.
* Significant difference from the initial state; #
significant difference from the control (p < 0.05).
Effects of the galanin peptides on the Cu,Zn-SOD, GSH-Px, and CAT activities in vitro. The myocardial reperfusion injury accompanied by the disruptions in the structure of cardiomyocyte membranes [15] can facilitate transfer of the exogenous galanin peptides from the blood flow into the intracellular space. This does not exclude direct interaction of the peptides with the antioxidant enzymes of the myocardium. We have modeled such situation by incubation of the G1, G2, and Gal peptides with commercial preparations of the Cu,Zn-SOD, GSH-Px, and CAT enzymes in model systems in vitro. Choosing the peptide concentration in the incubation medium was based on the following considerations. The optimal dose of G1 and G2 for intravenous injection to a rat is 1 mg/kg, and it is 0.5 mg/kg for Gal. Taking into account molecular mass of the peptides (Table 1) and average volume of the circulating blood (20 ml) in a rat with average mass of the body (350 g), we have the resulting peptide concentration in the blood flow equal to 0.012 mM for G1 and G2 and to 0.003 mM for Gal. Therefore, for assessment of the effect of peptide on activities of the antioxidant enzymes in the incubation medium we used two concentrations of the peptides: one close to the physiological concentration (0.01 mM) and another one order of magnitude higher (0.1 mM) (Table 3). It follows from the results presented in Table 3 that the G1 and Gal peptides did not affect activities of the antioxidant enzymes during the long-term incubation (the differences between the experimental and control groups were statistically insignificant). At the same time, G2 at the abovementioned concentrations caused noticeable activation of Cu,Zn-SOD activity (by 24 and 34%, respectively), insignificantly decreased CAT activity (on average, by 4.5% independent of concentration), and did not affect activity of GSH-Px. However, considering that the peptide concentrations used in these model experiments and duration of their interaction with the enzymes are unlikely the same as in vivo conditions, it follows from these results that G1, G2, and Gal practically do not affect activities of the antioxidant enzymes.
Table 3. Effects of the galanin peptides on
activities of the antioxidant enzymes in vitro
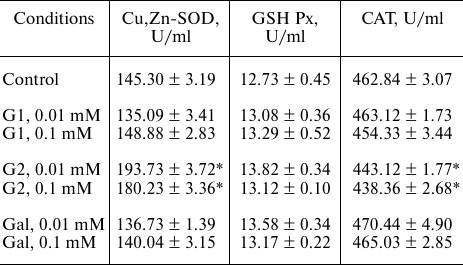
Note. Data are presented for the series of three experiments.
* Significant difference from the control (p < 0.05).
Effect of the peptides on the free-radical oxidation of human blood plasma LDL. To study the effect of G1, G2, and Gal on the parameters of free radical lipid oxidation, we used the previously developed kinetic method of studying inhibitory activity of the compounds in the model of Cu2+-initiated oxidation of natural LDL isolated from the blood plasma of healthy donors [13]. It is known that duration of the induction period τ (lag-phase of oxidation) is determined by the following equation: τ = [InH]/w, where [InH] is concentration of inhibitors of free radical processes in the system, and w is rate of oxidation initiation [16, 17]. We performed experiments using the same specimens of freshly isolated LDL under standard conditions. Obviously, concentration of the natural endogenous inhibitors of free radical processes [InH] in the initial specimens of LDL was identical. The initiation rate (w) of oxidation of LDL isolated from blood plasma also has to be standard, because it depends on the contents of the in vivo produced lipohydroxyperoxides [LOOH]. In the presence of copper ions lipohydroxyperoxides are destroyed with production of active lipid radicals initiating further oxidation of unsaturated substrates, polienic lipids [18, 19]:
LOOH + Cu2+→LO2· + H+ + Cu+
LOOH + Cu+→LO· + OH– + Cu2+
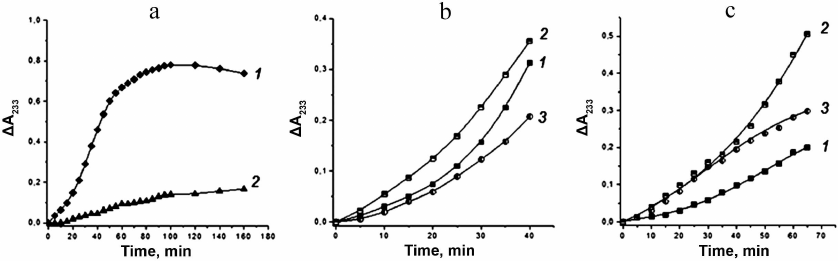
Thus, duration of the induction period of LDL oxidation (τ) in our experiments depended only on the antioxidant ability of the exogenous peptides introduced into the oxidation medium. Results of the kinetic experiments are presented in Fig. 3. These results demonstrate a pronounced inhibitory action of the synthetic phenolic antioxidant, BHT, at concentration of 0.01 mM (Fig. 3a). The peptides G1, G2, and Gal at concentrations of 0.01 mM and 0.1 mM also suppressed the free radical oxidation of LDL (Fig. 3, b and c).
Fig. 3. Characteristic kinetic curves of Cu2+-initiated free radical oxidation of LDL in the presence of the synthetic phenolic antioxidant BHT and of the G1, G2, and Gal peptides. a) Effect of BHT: curve 1 presents control (without additions); curve 2 in the presence of 0.01 mM BHT. Effect of peptides: b) in the presence of G1 (curve 1), G2 (curve 2), and Gal (curve 3) at concentration of 0.01 mM; c) in the presence of G1 (curve 1), G2 (curve 2), and Gal (curve 3) at concentration of 0.1 mM.
The induction period values (τ) calculated from the oxidation kinetic curves are presented in Table 4. It follows from these τ values that all studied galanin peptides possess an inhibitory activity with respect to the free radical oxidation of lipids. Duration of the induction periods of oxidation for G1, G2, and Gal at concentration of 0.01 mM was significantly higher than in the control (by 45, 15, and 75%, respectively). Increase of the peptide concentration in the incubation medium to 0.1 mM led to 2.75-fold increase in the antioxidant activity of G1 and to 1.5-fold increase in the activities of G2 and Gal as compared to the control, as well as to the 25% decrease in the induction period for Gal as compared to the one at concentration of 0.01 mM. The results of experiments with different concentrations of the peptides demonstrated that the ability of these compounds to inhibit free radical oxidation of lipids decreased in the following order: G1 > Gal > G2.
Table 4. Duration of the induction periods
of free radical oxidation of LDL (τ, min)
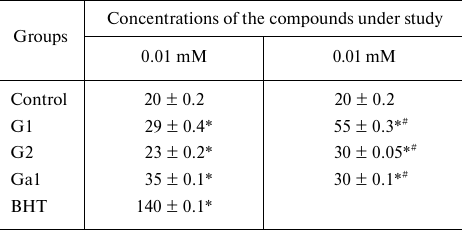
Note. * p < 0.05 as compared to the control; #
p < 0.05 as compared to the peptide at concentration of 0.01
mM; n = 3.
DISCUSSION
Antioxidant activities of the full-size galanin Gal and its natural and chemically modified fragments (G1 and G2, respectively) have been demonstrated in the present work in two models of oxidative stress: the myocardial I/R injury in vivo and the free radical oxidation of human blood plasma LDL in vitro. Intravenous injection of the galanin peptides to rats after the regional ischemia reduced irreversible damage of the AAR. This was manifested by reduction of the myocardium infarction size and by protection of the cardiomyocyte membranes – the decrease in the activities of CK-MB and LDH in the blood plasma at the end of reperfusion. The G1, G2, and Gal peptides decreased production of the spin adduct of hydroxyl radicals DMPO-OH in the interstitium of the AAR at the end of reperfusion, G2 and Gal also decreased generation of the secondary LDL products in the reperfused myocardium. Moreover, all galanin peptides inhibited the Cu2+-induced free radical oxidation of human LDL in vitro.
As it has been mentioned above, injection of the G1, G2, and Gal peptides to rats after the period of regional ischemia of the myocardium decreased production of DMPO-OH in the AAR of the LV during recovery of the blood flow. Generation of this spin adduct in the dialysis fiber seemed to be a consequence of penetration of hydroxyl radicals produced during the Fenton’s reaction into it. It is also likely that the DMPO-OH was generated outside of the dialysis fiber: in this case, the DMPO molecules could penetrate into myocardial tissue along the concentration gradient through the pores in the dialyzer membrane, and after the interaction with ROS in the cardiac muscle tissue the generated spin adduct could return into the dialyzer. Interaction of the spin-trapping DMPO molecules with superoxide anion-radicals with generation of the DMPO-OOH adduct and its subsequent spontaneous conversion to the hydroxide adduct also cannot be ruled out [20]. Nevertheless, the results obtained by microdialysis method indicated decrease in the ROS generation in the reperfused myocardium under the effect of the peptides. These results are in agreement with the ability of the G1 peptide to decrease generation of the superoxide-anion and hydrogen peroxide in mitochondria of isolated cardiomyocytes incubated under reoxygenation conditions after the period of hypoxia [3]. It is known that hyperproduction of ROS is one of the major mechanisms of myocardium damage during reperfusion leading to the increase of cardiomyocyte membrane permeability, changes in the ionic balance, and the cell death via necrosis and apoptosis [21]. As a result, the decrease in the DMPO-OH production in the experimental groups, which received injections of the G1, G2, and Gal peptides, was accompanied by the noticeable decrease in the size of myocardial infarction and by the decrease in activities of the necrosis markers in the blood plasma at the end of reperfusion (Fig. 1). Moreover, the decrease in the contents of LPO products to pre-ischemic values in the AAR of the LV was detected in the animals protected by the injections of G2 or Gal (Table 2). These data unambiguously indicate that the galanin peptides are able to reduce oxidative stress during reperfusion.
We showed previously that the subcutaneous injections of the G2 peptide to rats with doxorubicin-caused cardiomyopathy during 8 weeks decreased LPO and increased activities of Cu,Zn-SOD and GSH-Px in the damaged heart [6]. In the present work no significant effects of the G1, G2, and Gal peptides on activities of the key antioxidant enzymes Cu,Zn-SOD, CAT, and GSH-Px in the AAR of rat myocardium were observed. Only slight increase in the activity of GSH-Px under the effect of G2 and Gal and decrease in the activity of Cu,Zn-SOD under the effect of G1 was detected (Table 2). It is very likely that the short-term reperfusion (1 h) is insufficient for inducing expression of the genes of these enzymes in the myocardium. The results of our work [6] and the study by Timotin et al. [22] in which the decrease in the myocardium infarction size in mice as a result of the long-term injections of Gal was accompanied by the increase in Cu,Zn-SOD mRNA in the cardiomyocytes speak in favor of this assumption. The effects of G1, G2, and Gal on activities of the commercial antioxidant enzymes in vitro were assessed to understand the causes of changes in the activities of Cu,Zn-SOD and GSH-Px in the AAR of the rat heart under the effects of peptides during the reperfusion. Despite the long-term incubation (24 h) and the use of high concentrations of the peptides (0.1 mM), a noticeable effect was detected only for G2, which was manifested as activation of Cu,Zn-SOD and inhibition of CAT (Table 3). Different effects of the galanin peptides on the activities of Cu,Zn-SOD, CAT, and GSH-Px in the in vitro and in vivo experiments imply that their ability to decrease generation of ROS and LPO products in the rat heart during reperfusion and their action on the antioxidant enzymatic system are not related.
It is known that many peptides are able to scavenge ROS and inhibit lipid peroxidation [23, 24]. However, to the best of our knowledge, there are no literature data on the direct antioxidant action of galanin or its biologically active N-terminal fragments. Therefore, studies on mechanisms of reducing oxidative stress in the heart with the galanin peptides seem to be an important task for future investigations. In this connection the results of the studies on the effects of G1, G2, and Gal on the Cu2+-induced free radical oxidation of human blood plasma LDL in vitro are interesting. Our results demonstrated inhibitory effect of these compounds associated with the decrease of production of the lipid radicals, although this effect was weaker than the effect of synthetic antioxidant BHT (Table 4, Fig. 3). Nevertheless, it cannot be ruled out that suppression of the lipid free radical oxidation in this model could be caused by chelation of copper ions by the studied peptides.
In addition to regulation of the free radical processes, activation of different transduction pathways via binding of the galanin peptides to the GalR1-3 receptors also can contribute to the reduction of the cell damage. All three subtypes of galanin receptors inhibit activity of adenylate cyclase through the Gi/Go proteins that leads to inhibition of the transcription factor CREB – a protein binding the cyclo-AMP-dependent element. This increases expression of the glucose transporter GLUT4 and is favorable for its displacement into sarcolemma stimulating glucose uptake and oxidation by cardiomyocytes. Triggering of this mechanism is vital under conditions of decreased ATP production [25]. Coupling of the GalR2 receptor with the protein Gq/11 activates phospholipase C and through hydrolysis of phosphatidylinositol diphosphate regulates calcium homeostasis that improves inotropic state of the heart. The lower links of this signaling pathway cause phosphorylation of the protein kinase B (Akt), inhibition of proapoptotic proteins BAD/BAX, caspase-3, and caspase-9 [1, 26]. As a rule, the decrease in apoptosis of cardiomyocytes in the in vivo models is accompanied by limited myocardium infarction area and improvement of the heart contractility [27]. Activation of GalR1 and GalR2 stimulates the signaling pathways initiated by the mitogen-activated protein kinases (MEK1/2 and ERK1/2) leading to inhibition of the opening of the mitochondrial permeability transition pore (mPTP) and thus is favorable for survival and motility of the cells [28]. Moreover, activation of phosphorylation of the ERK kinases contributes to the increase in expression of the receptors activated by the peroxisome proliferators (PPARs), which control energy metabolism including also expression of PPARγ that stimulates uptake and oxidation of glucose by cardiomyocytes [29]. As a consequence, the peptide agonists of galanin receptors are able to enhance adaptation mechanisms of the metabolic defense during the heart damage.
An important aspect of the action of bioactive peptide is their effects on the intensity of free radical oxidation in organs and tissues, which is accompanied by correction of the disorders caused by various pathologic factors. In this connection, natural and synthetic peptide regulators may be considered as promising pharmacological agents promoting reduction of the stress-induced changes in the organism. The obtained results demonstrate involvement of the galaninergic system in the heart adaptation to the ischemia/reperfusion injury and oxidative stress. These data indicate potential of the molecular design of the pharmacological agonists of galanin receptor GalR2 with improved physicochemical characteristics (solubility, proteolytic stability) and comprehensive study on their mechanisms of action. Such compounds can be used as a basis for development of a new class of cardioprotectors for the therapy of various cardiovascular diseases.
Funding. The work was financially supported by the Russian Foundation for Basic Research (projects nos. 18-015-0008-a and 18-015-0009-a).
Ethics declarations. The authors declare no conflict of interest in financial or any other sphere. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
1.Branchek, T. A., Smith, K. E., Gerald, C., and
Walker, M. W. (2000) Galanin receptor subtypes, Trends Pharmacol.
Sci., 21, 109-117, doi: 10.1016/S0165-6147(00)01446-2.
2.Timotin, A., Pisarenko, O., Sidorova, M., Studneva,
I., Shulzhenko, V., et al. (2017) Myocardial protection from
ischemia/reperfusion injury by exogenous galanin fragment,
Oncotarget, 8, 21241-21252, doi:
10.18632/oncotarget.15071.
3.Pisarenko, O., Timotin, A., Sidorova, M., Studneva,
I., Shulzhenko, V., et al. (2017) Cardioprotective properties of
N-terminal galanin fragment (2-15) in experimental ischemia/reperfusion
injury, Oncotarget, 8, 60, 101659-101671, doi:
10.18632/oncotarget.21503.
4.Azmuko, A. A., Veselova, O. M., Molokoedov, A. S.,
Ovchinnikov, M. V., Palkeeva, M. E., et al. (2018) Tetradecapeptides
improving the reducing function of the cardiovascular system in
ischemia, Patent no. 2648846. RF. A61K 38/10 (2006.01).
5.Palkeeva, M., Studneva, I., Molokoedov, A.,
Serebryakova, L., Veselova, O., et al. (2019) Galanin/GalR1-3 system: a
promising therapeutic target for myocardial ischemia/reperfusion
injury, Biomed. Pharmacother., 109, 1556-1562, doi:
10.1016/j.biopha.2018.09.182.
6.Studneva, I. M., Veselova, O. M., Bakhtin, A. A.,
Konovalova, G. G., et al. (2020) The mechanisms of cardiac protection
using synthetic agonist of galanin receptors at the chronic damage
caused by chronic injections of doxorubicin, Acta Naturae,
12, 28-37, doi: 10.32607/actanaturae.10945.
7.Sidorova, M. V., Palkeeva, M. E., Avdeev, D. V.,
Molokoedov, A. S., Ovchinnikov, M. V., et al. (2020) Convergent
synthesis of the rat galanin and study of its biological activity,
Russ. J. Bioorg. Chem., 46, 32-42, doi:
10.1134/S1068162020010100.
8.Kitakaze, M., Takashima, S., Funaya, H., Minamino,
T., Node, K., et al. (1997) Temporary acidosis during reperfusion
limits myocardial infarct size in dogs, Am. J. Physiol.,
272, H2071-H2078, doi: 10.1152/ajpheart.1997.272.5.H2071.
9.Beauchamp, C., and Fridovich, I. (1971) Superoxide
dismutase: improved assays and assay applicable to acrylamide gels,
Anal. Biochem., 44, 276-287, doi:
10.1016/0003-2697(71)90370-8.
10.Aebi, H. (1984) Catalase in vitro,
Methods Enzymol., 105, 121-126, doi:
10.1016/s0076-6879(84)05016-3.
11.Lankin, V. Z., and Gurevich, S. M. (1976)
Inhibition of lipid peroxidation and detoxification of lipoperoxides by
protective enzyme systems (superoxide dismutase, glutathione
peroxidase, glutathione reductase) during experimental malignant
growth, DAN SSSR, 226, 705-708.
12.Draper, H. H., and Hadley, M. (1990)
Malondialdehyde determination as index of lipid peroxidation,
Methods Enzymol., 186, 421-431, doi:
10.1016/0076-6879(90)86135-i.
13.Lankin, V. Z., Kandalintseva, N. V., Konovalova,
G. G., Tikhase, A. K., Kholshin, S. V., et al. (2017) Express-screening
method of potential antioxidants using a kinetic model of
copper-induced free radical oxidation of human blood plasma low density
lipoproteins, Patent on Invention RU 2629398.
14.Britigan, B. E., Cohen, M. S., and Rosen, G. M.
(1987) Detection of the production of oxygen-centered free radicals by
human neutrophils using spin trapping techniques: a critical
perspective, J. Leukoc. Biol., 41, 349-362, doi:
10.1002/jlb.41.4.349.
15.Vanden Hoek, T. L., Shao, Z., Li, C., Zak, R.,
Schumacker, P. T., and Becker, L. B. (1996) Reperfusion injury on
cardiac myocytes after simulated ischemia, Am. J. Physiol.,
270, H1334-H1341, doi: 10.1152/ajpheart.1996.270.4.H1334.
16.Emanuel, N. M., and Lyaskovskaya, Y. N. (1961)
Inhibition of Lipid Oxidation Processes, Pischepromizdat (in
Russian), Moscow, pp. 10-19.
17.Lankin, V., Konovalova, G., Tikhaze, A., Shumaev,
K., Kumskova, E., and Viigimaa, M. (2014) The initiation of free
radical peroxidation of low-density lipoproteins by glucose and its
metabolite methylglyoxal: a common molecular mechanism of vascular wall
injure in atherosclerosis and diabetes, Mol. Cell. Biochem.,
395, 241-252, doi: 10.1007/s11010-014-2131-2.
18.Lankin V. Z. (2003) The enzymatic systems in the
regulation of free radical lipid peroxidation, in Free Radicals,
Nitric Oxide, and Inflammation: Molecular, Biochemical, and Clinical
Aspects, NATO Science Series (Tomasi, A., et al., eds) Vol. 344,
IOS Press, Amsterdam, pp. 8-23.
19.Lankin, V. Z., Antonovsky, V. L., and Tikhaze, A.
K. (2004) Regulation of free radical lipoperoxidation and organic
peroxides metabolism during normal station and pathologies, in
Peroxides at the Beginning of the Third Millennium (Antonovsky,
V.L., et al., eds) Nova Science Publishers Inc., New York, pp.
85-111.
20.Timoshin, A. A., Drobotova, D. Y., Tskitishvili,
O. V., Serebryakova, L. I., Pisarenko, O. I. et al. (2010) Protective
effect of dinitrosylic iron complexes with glutathione under condition
of regional ischemia of rat myocardium: study by microdialysis method,
Rep. Acad. Sci. (Section Biophysics), 432, 416-419.
21.Murphy, E., and Steenbergen, C. (2008) Mechanisms
underlying acute protection from cardiac ischemia-reperfusion injury,
Physiol. Rev., 88, 581-609, doi:
10.1152/physrev.00024.2007.
22.Timotin, A., Cinato, M., Kramar, S., Loy, H.,
Merabishvili, G., et al. (2019) Galanin is a checkpoint regulator of
mitochondrial biogenesis coordinating a pro-survival phenotype in
post-infarct myocardial remodeling, Lancet, preprint, doi:
10.2139/ssrn.3424189.
23.Power, O., Jakeman, P., and FitzGerald, R. J.
(2013) Antioxidative peptides: enzymatic production, in vitro
and in vivo antioxidant activity and potential applications of
milk-derived antioxidative peptides, Amino Acids, 44,
797-820, doi: 10.1007/s00726-012-1393-9.
24.Jakubczyk, A., Karas, M., Rybczynska-Tkaczyk, K.,
Zielinska, E., and Zielinski, D. (2020) Current trends of bioactive
peptides – new sources and therapeutic effect, Foods,
9, 846, doi: 10.3390/foods9070846.
25.Tian, R., and Abel, E. D. (2001) Responses of
GLUT4-deficient hearts to ischemia underscore the importance of
glycolysis, Circulation, 103, 2961-2966, doi:
10.1161/01.CIR.103.24.2961.
26.Lang, R., Gundlach, A. L., Holmes, F. E., Hobson,
S. A., Wynick, D., et al. (2015) Physiology, signaling, and
pharmacology of galanin peptides and receptors: three decades of
emerging diversity, Pharmacol. Rev., 67, 118-175.
27.Krijnen, P. A., Nijmeijer, R., Meijer, C. J.,
Visser, C. A., Hack, C. E., and Niessen, H. W. (2002) Apoptosis in
myocardial ischaemia and infarction, J. Clin. Pathol.,
55, 801-811, doi: 10.1136/jcp.55.11.801.
28.Hausenloy, D. J., and Yellon, D. M. (2013)
Myocardial ischemia-reperfusion injury: a neglected therapeutic target,
J. Clin. Invest., 123, 92-100, doi: 10.1172/JCI62874.
29.Jay, M. A., and Ren, J. (2007) Peroxisome
proliferator-activated receptor (PPAR) in metabolic syndrome and type 2
diabetes mellitus, Curr. Diab. Rev., 3, 33-39, doi:
10.2174/157339907779802067.