Hill Reaction in Pea Chloroplasts: Contribution of Photosystem II and Photosystem I to Ferricyanide Reduction
V. D. Samuilov,1,2 E. L. Barsky,1 and A. V. Kitashov1
1Department of Cell Physiology and Immunology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3807.2To whom correspondence should be addressed.
Submitted March 19, 1997.
The effect of DNP-INT (an inhibitor of cytochrome b6f complex at the level of plastoquinol QZH2 oxidation) on O2 evolution by pea chloroplasts in the Hill reaction with ferricyanide as an electron acceptor was studied. The DNP-INT inhibitory effect on O2 evolution decreased with a decrease in light intensity. This indicates that ferricyanide is preferably reduced by photosystem (PS) II than PS I at low light. The inhibitory effect of DNP-INT in the Hill reaction with ferricyanide at saturating and non-saturating light intensity was not affected by antimycin A, an inhibitor of PS I-dependent cyclic electron transfer at the level of ferredoxin-plastoquinone reductase. The inhibitory effect of DNP-INT on electron transfer from H2O to ferricyanide decreased with an increase in ferricyanide concentration from 50 µM to 3 mM: the contribution of PS II to ferricyanide reduction increased with the increase of ferricyanide concentration. The effect of DNP-INT on the photoreduction of other Hill reagents (silicomolybdate, phenyl-p-benzoquinone, p-benzoquinone, duroquinone, vitamin K3, DPIP, TMPD, and DAD) was also investigated.
KEY WORDS: electron transfer, photosystem II, photosystem I, ferricyanide, DNP-INT, antimycin A, chloroplasts.
Abbreviations: DAD) diaminodurene (2,3,5,6-tetramethyl-p-phenylenediamine); DBMIB) 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone; DCMU) 3-(3,4-dichlorophenyl)-1,1-dimethylurea; DNP-INT) 2-iodo-6-isopropyl-3-methyl-2',4,4'-trinitrodiphenyl ether; DPIP) 2,6-dichlorophenolindophenol; HQNO) 2-n-heptyl-4-hydroxyquinoline-N-oxide; PS) photosystem; TMPD) N,N,N',N'-tetramethyl-p-phenylenediamine.
Artificial electron donors and acceptors are widely used in studies of
the electron transfer chain during oxygenic photosynthesis [1]. These compounds are capable of selectively turning
on separate parts of the photosynthetic chain. To turn on electron
transfer involving photosystem (PS)3 II (the Hill reaction)
one can use ferricyanide and p-benzoquinone and its derivatives
as reductant in illuminated thylakoids of chloroplasts and
cyanobacteria coupled with oxygen evolution. Redox mediators (TMPD,
DPIP, etc.) reduced by ascorbate support PS I dependent reduction of
NADP+ or O2 (the Mehler reaction) or
methylviologen. Most of the tested electron donors and acceptors are
not selective in interactions with components of the electron transfer
chain. For instance, ferricyanide can be reduced by PS II, as well as
by PS I [1-3]. Using DNP-INT
and DBMIB that suppress oxidation of plastoquinol
QZH2 in cytochrome b6f
complex [4-6] makes it possible
to determine the contribution of PS II and PS I to electron transfer
from H2O to ferricyanide and other acceptors [7, 8].
Rates of electron transfer through PS II or PS I depend on the relation between the number of reaction centers of the photosystems and the sizes of their light-harvesting complexes, on the spectral composition and intensity of the actinic light [2, 9, 10], and on the functional state of cytochrome b6f complex [3, 7]. In intact chloroplasts assimilating CO2, the extent of NADP+ photoreduction is higher at low light intensity than at high light intensity [11]. This result seemed unexpected inasmuch as the rate of turnover of the electron transfer chain, and, accordingly, the rate of O2 evolution and NADP+ photoreduction ought to be higher at high light intensity than at low light intensity. This effect was explained as the result of CO2 assimilation through the Calvin cycle being suppressed at low light due to ATP deficiency, this resulting in a decrease of NADPH utilization and the lack of NADP+ that leads to retardation of non-cyclic electron transfer from H2O. In contrast, the utilization of NADPH increases, the pool of NADP+ enlarges, and non-cyclic electron transfer from H2O is stimulated at high light, when the ATP pool is replenished via the PS I-dependent cyclic electron transfer chain [11]. Thus, the redox state of NADP+ in chloroplasts depends on the ATP/ADP ratio.
This paper reports data on ferricyanide reduction involving PS II and PS II + PS I at various rates of electron transfer chain turnover regulated by the intensity of the actinic light. Unlike NADP+, ferricyanide photoreduction does not depend on the system of CO2 assimilation and, therefore, on the ATP/ADP ratio. Nevertheless, ferricyanide is preferably reduced by PS II than by PS I as light intensity decreases. In addition to ferricyanide, some other electron acceptors were tested.
MATERIALS AND METHODS
Chloroplasts from the leaves of 10-12-day-old pea seedings were isolated in a solution containing 200 mM sucrose, 10 mM NaCl, and 20 mM Tricine-NaOH (pH 7.5), washed, and resuspended in the same buffer [12]. The chloroplast suspension was stored at 0°C and used during 3-4 h after isolation. Oxygen evolution was measured using the closed Clark-type Pt electrode. The current passed through the electrode was amplified and fed to an analog-to-digital data conversion card connected to a personal computer. The O2 activity in tested solutions was measured at 5 Hz and converted to a 14-bit integer with a resolution of 15 pmoles/ml. The rate of O2 activity change was determined from a linear regression equation given that the experimental data and linear model meet Fisher's criterion of concordance in a certain time interval. In most experiments, a white saturating light intensity of ~103 W/m2 was used. In some experiments, the light intensity was reduced so that the rate of the Hill reaction becomes 20-25% of that at the saturating light intensity. The incubation medium contained chloroplasts (10-12 µg chlorophyll/ml), 200 mM sucrose, 10 mM NaCl, 20 mM Tricine-NaOH (pH 7.5), 5 µM gramicidin D, and ferricyanide at concentrations given in figure captions. In experiments with silicomolybdate additions were made in the following order: silicomolybdate, gramicidin D, DCMU, and ferricyanide. Chlorophyll concentration was determined as described by Arnon [13].
RESULTS
In experiments with reagents accelerating de-activation of the water-oxidizing system (ADRY reagents), it was shown [14, 3] that the efficiency of these reagents to inhibit photosynthetic O2 evolution by chloroplasts and cyanobacterial thylakoids decreases upon an increase of the light intensity. However, the efficiency of DCMU to inhibit electron transfer from primary plastoquinone QA to secondary plastoquinone QB [15] and the efficiency of HQNO to inhibit oxidation of QBH2 by the membrane pool of plastoquinone QP [16, 17, 3] were not affected by changes in the light intensity [14, 3]. The change in the activity of ADRY reagents depending on the light intensity (i.e., on the rate of the electron transfer chain turnover) is due to their redox properties: they are oxidized at the electron donor branch of PS II [18] and reduced at the electron acceptor branch of PS II, competing with ferricyanide as electron acceptor at the level of plastoquinone QP [14, 3].
We studied the effect of DNP-INT on O2 evolution in the Hill reaction with ferricyanide. Like DBMIB [5, 6], DNP-INT [4] inhibits oxidation of plastoquinol QZH2 in the cytochrome b6f complex. Both compounds inhibit electron transfer from duroquinol (which reduces plastoquinone QZ) to methylviologen, but in the presence of DCMU it does not affect electron transfer from H2O to silicomolybdate (PS II activity) nor electron transfer from ascorbate + TMPD to methylviologen (PS I activity) [14, 3]. However, in contrast to DBMIB, which is capable of accepting electrons [19], DNP-INT does not have redox properties [20]. Because of this, DNP-INT was used in the study.
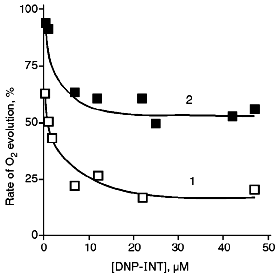
As can be seen from Fig. 1, the inhibitory effect of DNP-INT on O2 evolution by chloroplasts is significantly reduced as the light intensity is reduced, that is, at lower light intensity ferricyanide is preferably reduced by PS II rather than by PS I. Relative (normalized) rates of O2 evolution on the plateau of the titration curves are ~3 times higher at the low than at the high light intensity. The pattern is apparently the same with NADP+ whose level of reduction at the low light intensity is higher than at the high light intensity [11]. However, ferrocyanide, unlike NADPH, is not utilized in reactions of CO2 assimilation, and its redox state can not depend on ATP/ADP ratio.
PS I supports cyclic along with non-cyclic electron transfer (see [21] for a review). Under flash illumination, two phases of P700 (PS I reaction center) reduction were revealed in the dark [22, 23]. The microsecond phase of P700 reduction, as well as NADP+ reduction, was suppressed by DBMIB or DNP-INT, and this was caused by non-cyclic electron transfer from H2O [22]. The millisecond phase was far more resistant to these inhibitors and was caused by PS I-dependent cyclic electron transfer [22]. Under conditions of our experiments, cyclic electron transfer can compete for electrons with (PS II + PS I)-dependent ferricyanide reduction. Based on the data presented in Fig. 1, one could suggest that competition of cyclic electron transfer and ferricyanide photoreduction would be more exhibited at the high light intensity rather than at the low light intensity when ferricyanide was preferably reduced by PS II. In this connection, we tested the action of antimycin A inhibiting cyclic electron transfer involving PS I in chloroplasts on the level of ferredoxin:plastoquinone oxidoreductase [21].Fig. 1. Effect of DNP-INT on O2 evolution by pea chloroplasts incubated with ferricyanide at saturating (1) and non-saturating (2) light intensity. For conditions of incubation and illumination of chloroplasts see "Materials and Methods". The concentration of ferricyanide was 3 mM. The 100% rate of O2 evolution was 150 and 40 µmoles/h per mg chlorophyll at saturating and non-saturating light intensity, respectively.
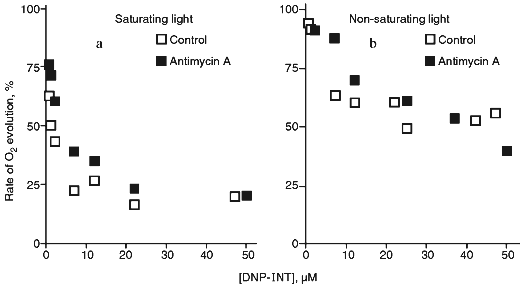
At elevated concentrations, antimycin A exhibits an uncoupling effect in chloroplasts [24], chromatophores of purple bacteria [25], and mitochondria [26] (therefore, experiments were performed in the presence of gramicidin D forming ion channels in the membranes), and, in addition, exhibits properties of a weak ADRY reagent [27]. Antimycin A, when added at 4 µM, had practically no affect of the inhibition by DNP-INT at the saturating as well as at the non-saturating light intensity (Fig. 2). Similar data (not shown) were obtained with 2 µM antimycin A. Thus, PS I-dependent cyclic electron transfer is not a factor to determine the rate of ferricyanide photoreduction.
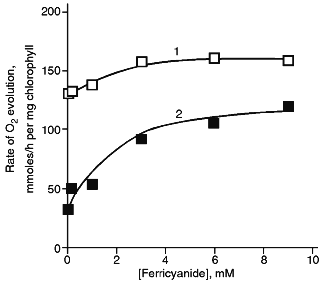
The rate of oxygen evolution by illuminated chloroplasts increased by 20-25% as the ferricyanide concentration was increased from 50 µM to 3 mM; it did not change with further increase in the ferricyanide concentration (Fig. 3). In chloroplasts incubated in the presence of DNP-INT, rates of O2 evolution increased 3.5-4 times as ferricyanide concentration increased, i.e., the level of the DNP-INT inhibitory effect on the electron transfer from H2O to ferricyanide decreased as the ferricyanide concentration increased. This implies that PS II contribution to ferricyanide reduction increases as the ferricyanide concentration in the chloroplast incubation medium is increased.Fig. 2. Effect of DNP-INT on O2 evolution by pea chloroplasts incubated with ferricyanide and antimycin A at saturating and non-saturating light intensities. The concentration of antimycin A was 4 µM. Other conditions as in Fig. 1.
The final series of experiments included testing a number of other electron acceptors. Accordingly to the data of [8, 14, 3], silicomolybdate photoreduction that is resistant to DCMU and is probably due to interaction between silicomolybdate and non-heme iron in the electron-acceptor branch of PS II [28] was not sensitive to DNP-INT (Table 1). Electron transfer from H2O to phenyl-p-benzoquinone was inhibited by DCMU (data not shown), but was almost insensitive to DNP-INT. However, a number of quinones was reduced by PS I [29]. Indeed, photoreduction of p-benzoquinone, duroquinone, and vitamin K3 was inhibited by DNP-INT by 40-50% (Table 1). Non-quinone compounds were also reduced by either PS II or PS I; the sensitivity of the process decreased in the order: DPIP, TMPD, DAD.Fig. 3. Rate of O2 evolution (1) and inhibitory effect of DNP-INT on O2 evolution (2) by pea chloroplasts as functions of ferricyanide concentration at saturating light. The 100% rate of O2 evolution was 130 and 28 µmoles/h per mg chlorophyll in the control and in the presence of 25 µM DNP-INT, respectively. Other conditions as in Fig. 1.
TABLE 1. Effect of DNP-INT (25 µM) on
O2 Evolution by Pea Chloroplasts with Ferricyanide (1.5 mM)
and Other Electron Acceptors (100 µM) Added in Combination with
Ferricyanide (1.5 mM) at Saturating Light Intensity

DISCUSSION
The data concerning the action of DNP-INT, an inhibitor of cytochrome b6f complex on the level of plastoquinol QZH2 oxidation [14], show that the rate of ferricyanide reduction by PS II is lower than that by PS I at the non-saturating light intensity (Fig. 1). In contrast, ferricyanide was reduced by PS I at a higher rate than that by PS II at saturating light intensity. As shown earlier [11], NADP+ photoreduction rate in chloroplasts is higher at low rather than at high light intensity. According to the idea of sequential participation of PS II, cytochrome b6f complex, and PS I in non-cyclic electron transfer from H2O to NADP+, the higher rate of NADP+ photoreduction at the low light intensity was explained by ATP deficiency which retards NADP+ regeneration in CO2 reduction reactions through the Calvin cycle. The decrease in the NADPH level with an increase in the light intensity was explained as stimulation of photophosphorylation as a result of cyclic electron transfer involving PS I [11].
However, as Fig. 2 shows, PS I-dependent cyclic electron transfer inhibited by antimycin A has no appreciable effect on ferricyanide reduction at the saturating and non-saturating light intensities. Therefore, the decrease in the NADPH level with an increase in the light intensity can be due not to filling the ATP pool from cyclic phosphorylation involving PS I, but to the difference in the rates of PS I and PS II turnover. Under the conditions of limiting light intensity, the rate of PS II-dependent non-cyclic electron transfer is 1.5-1.8 times higher than the rate of PS I-dependent non-cyclic electron transfer [2]. The increase of the sensitivity of ferricyanide photoreduction to DNP-INT with an increase in the light intensity (Fig. 1) indicates that under these conditions the rate of PS I turnover in non-cyclic electron transfer increases, and, accordingly, its role (together with cytochrome b6f complex, see [21] for a review) in generating the membrane potential and ATP synthesis also increases. The increase of the NADPH level [11], as well as the decrease of the sensitivity of ferricyanide photoreduction to DNP-INT (Fig. 1) with the decreased light intensity might be explained from NADP+ and ferricyanide reduction through PS II without the participation of PS I (see [30] for a review on direct reduction of NADP+ by PS II).
PS II reduces NADP+ and ferredoxin [31]. A mutant of Chlamydomonas reinhardtii lacking PS I is capable of phototrophic CO2 assimilation and performs light-dependent O2 and H2 evolution in the absence of CO2 [32]. There are data on direct Mn-dependent ferredoxin photoreduction by pheophytin in PS II [33] and on NADP+ photoreduction in isolated reaction center complexes of PS II with 1,5-diphenylcarbazide as an electron donor [34]. Generation of membrane potential in chromatophores of the purple bacterium Rhodospirillum rubrum incubated with TMPD and ascorbate was suppressed under anaerobic conditions due to over-reduction of components of the electron transfer chain [35]. This inhibitory effect was removed by methylviologen (E0' = -0.44 V); this is evidence of a redox interaction of methylviologen with the photosynthetic chain of R. rubrum (E0' of the secondary quinone is ~0 V) [35].
Thus, cyclic electron transfer involving PS I has practically no effect on ferricyanide reduction. This is consistent with the assumption that the maximum rate of the cyclic process does not exceed 3% of the non-cyclic process [21].
The contribution of PS I and PS II to ferricyanide reduction depends upon the concentration of ferricyanide added. Judging from the DNP-INT inhibitory effect (Fig. 3), the contribution of PS II to ferricyanide reduction increases as ferricyanide concentration increases; this agrees with the data [2]. These data seem to reflect the particular properties of spatial distribution of PS II and PS I in thylakoid membranes of chloroplasts (see [36] for a review). PS I is localized preferably in the peripheral regions of the grana and in the lamellae of stroma which is apparently more accessible for hydrophilic ferricyanide than is PS II. Only when ferricyanide concentration increases, it can get into the internal area of the grana (into the intrathylakoid volume); that makes it more accessible for plastoquinone QP, which is reduced by PS II.
Differences in the rates of oxygen evolution in the presence of quinone and non-quinone electron acceptors (Table 1) might be due to: 1) their hydrophilic/hydrophobic properties; 2) their tendency for autoxidation by oxygen, although the process is considerably inhibited by the ferricyanide added; 3) their affinity for binding sites. Besides, some quinones, particularly vitamin K3, induce strong quenching of the excited state of chlorophyll [37, 38]. Inasmuch as photoreduction of phenyl-p-benzoquinone was not sensitive to DNP-INT, but was inhibited by DCMU, one could suppose that phenyl-p-benzoquinone is preferably reduced by PS II, most likely at the level of QB. There are similar data for 2,6-dichloro-p-benzoquinone and 2,5-dimethoxy-3,6-dichloro-p-benzoquinone [39].
LITERATURE CITED
1.Hauska, G. (1977) in Encyclopedia of Plant
Physiology, Vol. 5, Photosynthesis I (Trebst, A., and Avron,
M., eds.) Springer-Verlag, Berlin, pp. 253-265.
2.McCauley, S. W., Taylor, S. E., Dennenberg, R. J.,
and Melis, A. (1984) Biochim. Biophys. Acta, 765,
186-195.
3.Samuilov, V. D., Renger, G., Pashenko, V. Z.,
Oleskin, A. V., Gusev, M. V., Gubanova, O. N., Vasil'ev, S. S., and
Barsky, E. L. (1995) Photosynth. Res., 46, 455-465.
4.Malkin, R. (1986) FEBS Lett., 208,
317-320.
5.Jones, R. W., and Whitmarsh, J. (1988) Biochim.
Biophys. Acta, 933, 258-268.
6.Rich, P. R., Madgwick, S. A., and Moss, D. A.
(1991) Biochim. Biophys. Acta, 1058, 312-328.
7.Böhme, H., Reimer, S., and Trebst, A. (1971)
Z. Naturforsh., 26b, 341-352.
8.Barr, R., and Crane, F. L. (1981) Plant
Physiol., 67, 1190-1194.
9.Melis, A., and Anderson, J. M. (1983) Biochim.
Biophys. Acta, 724, 473-484.
10.Bose, S., and Ramanujam, P. (1984) Biochim.
Biophys. Acta, 764, 40-45.
11.Takahama, U., Shimizu-Takahama, M., and Heber, U.
(1981) Biochim. Biophys. Acta, 637, 530-539.
12.Barsky, E. L., Gubanova, O. V., and Samuilov, V.
D. (1991) Biokhimiya, 56, 434-438.
13.Arnon, D. I. (1949) Plant Physiol.,
24, 1-15.
14.Samuilov, V. D., and Barsky, E. L. (1993) FEBS
Lett., 320, 118-120.
15.Böger, P., and Sandman, G. (1990) in
Controlled Release, Biochemical Effects of Pesticides, Inhibition of
Plant Pathogenic Fungi, Vol. 6, Chemistry of Plant
Protection (Haug, G., and Hoffmann, H., eds.) Springer-Verlag,
Berlin, pp. 174-216.
16.Barton, J. R., MacPeek, W. A., and Cohen, W. S.
(1983) J. Bioenerg. Biomembr., 15, 93-104.
17.Cohen, W. S., and Barton, J. R. (1983) Z.
Naturforsch., 38c, 793-798.
18.Samuilov, V. D., Barsky, E. L., Gubanova, O. N.,
Klimov, V. V., and Kozlov, Yu. N. (1995) FEBS Lett.,
357, 55-57.
19.Barsky, E. L., and Samuilov, V. D. (1992)
Vestnik Mosk. Univ. Ser. 16. Biol., 1, 20-22.
20.Haehnel, W., and Trebst, A. (1982) J.
Bioenerg. Biomembr., 14, 181-191.
21.Bendall, D. S., and Manasse, R. S. (1995)
Biochim. Biophys. Acta, 1229, 23-28.
22.Rurainski, H. J., Markgraf, T., and Borchert, S.
(1985) Biochim. Biophys. Acta, 809, 452-455.
23.Bottin, H., and Mathis, P. (1987) Biochim.
Biophys. Acta, 892, 91-98.
24.Drechsler, Z., Nelson, N., and Neumann, J. (1969)
Biochim. Biophys. Acta, 189, 65-73.
25.Remennikov, V. G., and Samuilov, V. D. (1979)
Biochim. Biophys. Acta, 548, 216-223.
26.Azzone, G. F., Pozzan, T., and Di Virgilio, F.
(1979) J. Biol. Chem., 254, 10206-10212.
27.Yerkes, C. T., and Crofts, A. R. (1992) in
Research on Photosynthesis (Murata, N., ed.) Vol. II, Kluwer,
Dordrecht, pp. 635-638.
28.Schansker, G., and Van Rensen, J. J. S. (1993)
Photosynth Res., 37, 165-175.
29.Izawa, S. (1980) Meth. Enzymol.,
69, 414-434.
30.Prince, R. C. (1996) Trends Biochem. Sci.,
21, 121-122.
31.Arnon, D. I. (1995) Photosynth Res.,
46, 47-71.
32.Greenbaum, E., Lee, J. W., Tevault, C. V.,
Blankinship, S. L., and Mets, L. J. (1995) Nature, 376,
438-441.
33.Allakhverdiev, S. I., and Klimov, V. V. (1992)
Z. Naturforsch., 47c, 57-62.
34.Arnon, D. I., and Barber, J. (1990) Proc.
Natl. Acad. Sci. USA, 87, 5930-5934.
35.Remennikov, V. G., and Samuilov, V. D. (1979)
Biochim. Biophys. Acta, 546, 220-235.
36.Melis, A. (1991) Biochim. Biophys. Acta,
1058, 87-106.
37.Karukstis, K. K., Boegeman, S. C., Fruetel, J.
A., Gruber, S. M., and Terris, M. H. (1987) Biochim. Biophys.
Acta, 891, 256-264.
38.Lee, J. W., Zipfel, W., and Owens, T. G.
(1992) J. Luminescence, 51, 79-89.
39.Sarojini, G., and Daniell, H. (1981) Z.
Naturforsch., 36c, 656-661.