Photophosphorylation in Alkalophilic Halobacterial Cells Containing Halorhodopsin: Chloride-Ion Cycle?
A. V. Avetisyan, A. D. Kaulen, V. P. Skulachev*, and B. A. Feniouk
Department of Bioenergetics, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3188; E-mail: skulach@head.genebee.msu.su* To whom correspondence should be addressed.
Received December 10, 1997; Revision received January 30, 1998
Light-driven ATP synthesis is found in cells of the alkalophilic bacterium Natronobacterium pharaonis containing halorhodopsin but deficient in H+-pumping bacteriorhodopsin. Photophosphorylation occurs with cyanide-inhibited respiratory chain as well as without cyanide in conditions with low Cl- concentration in the incubation medium. Increase in Cl- concentration from 0.1 to 2.35 M in the incubation medium leads to inhibition of photophosphorylation. Continuous illumination increases membrane DeltaPsi if respiration is inhibited by cyanide. This effect is stimulated by DCCD, an ATPase inhibitor. These data can be explained if one suggests that halorhodopsin pumps Cl- into the cells whereas Cl- release from the cells through Cl--ATP-synthase is coupled with the ATP synthesis (chloride-ion cycle).
KEY WORDS: chloride-ion cycle, halorhodopsin, alkalophilic bacterium, Cl--ATP-synthase
Abbreviations: DeltaµH+) transmembrane difference in electrochemical potential of H+ ions; DeltaµCl-) transmembrane difference in electrochemical potential of Cl- ions; DeltaPsi) transmembrane difference in electrical potential; DCCD) N,N´) dicyclohexylcarbodiimide; TPP+) tetraphenylphosphonium; TCA) trichloroacetic acid; FCCP) p-trifluoromethoxyphenylhydrazone; EDTA) ethylenediamine tetraacetic acid.
Natronobacterium pharaonis is an alkalophilic halophilic archaeon
that dwells in soda lakes in Kenya and Egypt where NaCl concentration
reaches 4 M and pH 9-10 [1]. Besides a respiration
chain, N. pharaonis has photoenergetics and photoreception
systems. There are two membrane proteins containing retinal,
halorhodopsin and phoborhodopsin [2].
Phoborhodopsin is a sensor protein that determines the repellent effect
of short wavelength light. Halorhodopsin transports Cl- from
outer medium into the cytoplasm using the energy of light [3, 4]. It is considered that the
main function of halorhodopsin is to maintain high intracellular
Cl- concentration. Furthermore, being an electrogenic ion
pump, halorhodopsin may participate in light-dependent ATP
synthesis.
If ATP synthesis of N. pharaonis is driven by electrochemical proton potential (DeltaµH+), then unfavorable DeltapH on the coupling membrane must be compensated by a large electrical component of DeltaµH+. At intracellular pH 7.5 and extracellular pH 9.5, according to Nernst equation, there must be an H+ diffusion potential of 120 mV. If H+ is the coupling ion, then it is necessary to generate DeltaPsi about 300 mV to maintain DeltaµH+ sufficient for ATP synthesis. This potential must be maintained by respiratory chain enzymes [5] and also by halorhodopsin pumping Cl- into the cell in presence of light.
Another possibility was pointed out by M. Engelhard (personal communication). He proposed that DeltaµCl- generated by halorhodopsin could be consumed by a hypothetical Cl--ATPase without a proton cycle. In that case adaptation to alkali environment can be realized not only by way of changing H+ to Na+ as the coupling ion [6], but also H+ to Cl-. The idea that Na+ is not a unique alternative coupling ion was recently postulated by Broun, whose data suggest the presence of Ca2+ cycle in cyanobacteria [7].
The aim of this work was to investigate the possible role of halorhodopsin in membrane potential generation and ATP synthesis in N. pharaonis in light of the Cl- cycle hypothesis.
MATERIALS AND METHODS
Strain and growth conditions. Natronobacterium pharaonis strain SP1/28 was a generous gift of M. Engelhard (Max Planck Institute of Molecular Physiology, Dortmund, Germany). Cells were grown in medium containing 3.4 M NaCl, 175 mM Na2CO3, 100 mM sodium acetate, 10 mM sodium citrate, 27 mM KCl, 4 mM MgSO4, 2 mM Na2HPO4, 4 µM CuSO4, 5 µM FeSO4, 4 µM MnCl2, 3 µM CaCl2, 3 µM ZnSO4, 0.75% casamino acids, 1% yeast extract, pH 9.5 [8]. Cells were grown in constant light (4 mW/cm2) at 41°C in Erlenmeyer flasks. Flask volume to culture volume ratio was 5:1. Cells were grown 3-4 days to apparent optical density 3.0 at 600 nm wavelength.
Membrane fraction preparation. Cells from 4 liters of culture were harvested, washed with solution containing 4 M NaCl and 50 mM Na2CO3, and suspended in the same solution to protein concentration of 15-20 mg/ml. Cells were treated with DNAse and were dialyzed against 40 volumes of water for 16 h and centrifuged at 12,100g for 10 min. The supernatant was centrifuged at 131,000g at 4°C for 1 h. The membrane pellet was suspended in 0.1 M NaCl. The suspension was centrifuged at 10,000g for 8 min to remove large membrane aggregates. Absorption spectra of N. pharaonis membranes had a distinct peak at 590 nm that disappeared after 0.5 M NH2OH treatment. Spectral changes specific for halorhodopsin response on actinic laser flash were also present.
Membrane potential measurements. Membrane potential was measured with a TPP+ selective electrode in an open thermostatted cell at 41°C with constant stirring. Protein concentration was 1 mg/ml, inner cell volume of N. pharaonis was taken as 1.5 µl/mg protein (M. Engelhard, personal communications). The electrode was calibrated by standard TPP+ solution; final TPP+ concentration was 4 µM. A 100 W halogen lamp was used for suspension illumination; light intensity was 11 mW/cm2. Potential calculations were made according to the modified Nernst equation:
Intracellular ATP concentration measurements. ATP was measured with the luciferin-luciferase method on an LKB 1251 luminometer. Cell suspension (1 mg/ml protein) was thermostatted at 41°C and constantly stirred on a magnetic stirrer. A 20 µl aliquot was taken and fixed in 1 ml of solution containing 2.5% TCA, 0.2 mM EDTA. After 10 min incubation a 20 µl aliquot of the solution obtained was added to 200 µl of 100 mM Tris-acetate (pH 7.75), 2 mM EDTA, and luminescence was measured for 10 sec after the injection of luciferin-luciferase reagent (firefly Photinus pyralis lantern extract). Protein concentration was determined according to the Lowry method.
Reagents. The following reagents were used in this work: casamino acids, firefly lantern extract in arsenate buffer for the luciferase method, ATP, and TPP were from Sigma (USA); yeast extract was from Difco (USA); EDTA, DNase, and DCCD were from Serva (Germany); NaCN was from Fluka (Switzerland).
RESULTS AND DISCUSSION
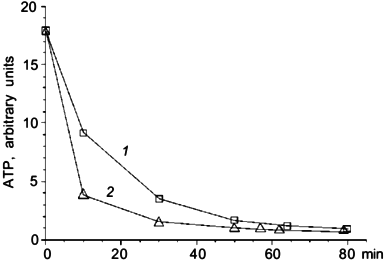
A series of experiments was made to estimate the role of halorhodopsin in ATP synthesis. We measured the intracellular ATP level in darkness and under illumination. Illumination has no influence on the ATP level in conditions when the respiratory chain was active. Inhibition of respiration by 5 mM NaCN led to ATP level decrease. Illumination considerably slowed this decrease (Fig. 1). The effect was not pH-dependent in the range of pH 8 to 10.5.
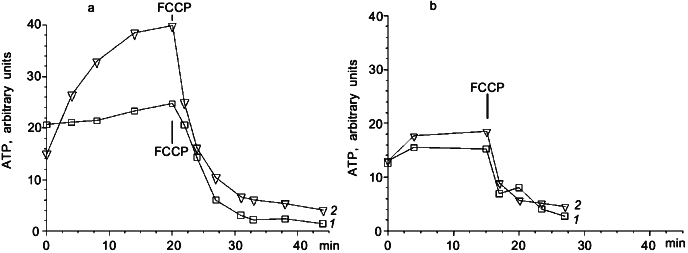
To clarify the role of Cl- in light-dependent ATP level maintenance, cells were suspended in a medium where Cl- concentration was lowered to 0.1 M. We added Na2SO4 to 1.5 M to compensate for the lower ionic strength. In these conditions there was an increase in ATP level in illuminated cells (Fig. 2a), which was not observed in darkness (Fig. 2b). There was no ATP level increase in cells suspended in 2.25 M NaCl medium (Fig. 2a). The effect of light shown on Fig. 2a was not pH dependent in the range from 8 to 10.5. Protonophoric uncoupler FCCP caused a sharp ATP level decrease in all experiments; however, in the medium with low Cl- concentration there was a low but measurable residual ATP level insensitive to FCCP, which was not observed in the medium with high Cl- concentration (Fig. 2a).Fig. 1. Effect of illumination on the rate of intracellular ATP level decrease in N. pharaonis. Cells are suspended in medium containing 4 M NaCl, 50 mM Na2CO3, pH 9.5, protein concentration is 1 mg/ml. Temperature is 41°C. At time zero NaCN (5 mM) is added to the suspension. 1) Illumination; 2) darkness.
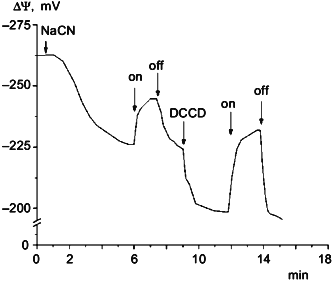
Measurements of DeltaPsi showed that in darkness under aeration the membrane potential was about 260-280 mV. In aerobic conditions illumination had no effect on the value of DeltaPsi. However, if membrane potential was decreased to 200-220 mV by cyanide or by anaerobiosis, then illumination caused DeltaPsi increase. This effect is shown in Fig. 3. The protonophoric uncoupler FCCP at 10 µM concentration caused DeltaPsi to fall to 180-190 mV and completely inhibited the light effect. In the medium with low Cl- concentration this light-dependent DeltaPsi increase was also observed only on the background of lowered DeltaPsi (data not shown).Fig. 2. Photophosphorylation in N. pharaonis cells. 1) Medium with high Cl- concentration: 2.25 M NaCl, 100 mM KCl, 50 mM Na2CO3, pH 8.5; 2) medium with low Cl- concentration: 1.5 M Na2SO4, 50 mM K2SO4, 0.1 M NaCl, 50 mM Na2CO3, pH 8.5. Temperature, 41°C. At time zero concentrated cell suspension is added to incubation media to final protein concentration 1 mg/ml. a) Incubation under illumination; b) incubation in darkness. Addition: 5 µM FCCP.
DCCD, an ATP-synthase inhibitor, increased the amplitude of DeltaPsi light responses (Fig. 3), but caused a DeltaPsi decrease in darkness; 50 µM DCCD caused intracellular ATP level to fall to zero. Probably, inhibition of ATPase by DCCD that caused the loss of ionic conduction made easier the generation of membrane potential by halorhodopsin due to the absence of ion current through the ATPase. The decrease of membrane potential after DCCD treatment in darkness indicates that there is ATPase contribution to membrane potential generation under these conditions.
The data suggest that halorhodopsin can serve as an energy supplier in N. pharaonis cells. But it is impossible to detect halorhodopsin contribution to ATP synthesis when the respiration chain is working because of relatively low power of halorhodopsin.Fig. 3. Cyanide, light, and DCCD effects on DeltaPsi in N. pharaonis cells. Medium contained: 2 M NaCl, 1 M Na2SO4, 50 mM Na2CO3, pH 9.5. Protein concentration was 1 mg/ml. Additions: 5 mM NaCN, 25 µM DCCD. On and off, light turned on and off.
Special attention should be drawn to the results shown in Fig. 2a. It is demonstrated that decrease in Cl- concentration in the incubation medium allows photophosphorylation in N. pharaonis cells to be observed. Under conditions mentioned illumination caused a 2.5-fold increase in intracellular ATP level.
These results could be anticipated if we presume that DeltaµCl- is generated by halorhodopsin and serves an energy source for a Cl--transporting ATP-synthase that allows Cl- outflow from the cell to the incubation medium. A decrease in outer Cl- concentration increases DeltapCl of favorable orientation. This must decrease DeltaPsi generated by Cl--pumping halorhodopsin. It seems that decrease of DeltaPsi, initially very high (actually close to the limit for biomembranes), decreases passive ion conductance of N. pharaonis membrane, and, therefore, lowers energy losses caused by this conductance. As a result there must be an increase in DeltaµCl-, that must increase ATP synthesis if it is catalyzed by a Cl--ATP-synthase.
An alternative explanation of N. pharaonis photophosphorylation mechanism is that DeltaPsi generated by halorhodopsin is consumed by a H+-ATP-synthase. But in that case the decrease of outer Cl- concentration must not facilitate, but restrain light-dependent ATP synthesis because it decreases DeltaPsi generated by halorhodopsin increasing DeltapCl component of DeltaµCl-.
Stimulation of ATP synthesis by lowering the outer Cl- concentration can hardly be explained by using DeltapH generated by a Cl-/OH- antiporter found in halobacteria [9]. In that case the antiporter would equalize Cl- and H+ concentration gradients. Then, having DeltapH about 2, the intracellular Cl- concentration should be about 40 mM (at extracellular Cl- concentration 4 M), which does not seem probable.
It can seem that the idea of Cl--ATP-synthase contradicts the fact that protonophoric uncoupler FCCP inhibits phosphorylation (Fig. 2a). This can be explained by deactivation of a H+-ATP-synthase, most probably also present in N. pharaonis, because of decrease in DeltaµH+ generated by the respiration chain. Moreover, in the presence of FCCP H+-ATP-synthase can consume ATP synthesized by Cl--ATP-synthase.
Cl--ATPase was previously described in a number of eucaryotes. For example, in leaf salt gland plasmalemma of a salt-tolerate plant Limonium vulgaris a Cl--ATPase that pumps Cl- out from the cytoplasm was discovered [10, 11]. A similar enzyme is pumping Cl- into intestine cells of the mollusk Aplysia californica [12-16]. It has molecular mass of 110 kD [12], is a P-type ATPase [13-15], and had been successfully reconstructed into liposomes [15, 16].
An F-type Cl--ATPase responsible for electrogenic Cl- import from medium into cell, capable of maintaining DeltaPsi at about 170 mV, was purified from Acetabularia acetabulum [17, 18]. The enzyme is composed of at least three subunits (a, b, and c with molecular masses 54, 50, and 40 kD, respectively) [19]. The amino acid sequence of subunit a is 77% identical to alpha-subunit of CF1 factor from spinach chloroplasts. As for subunit b, it is quite similar to the beta-subunit of CF1 factor from Acetabularia (92.5% identity) [20]. Cl--ATPase from Acetabularia acetabulum, as the one from Aplysia, was reconstituted into liposomes [21, 22]. Probably, the reversion of Cl--ATPase reaction that is catalyzed by enzymes of that kind, could explain light-dependent ATP synthesis in N. pharaonis cells.
The data obtained here can be considered as evidence in support of the existence of a Cl- cycle in nature, which could perform coupling of exergonic processes with ATP synthesis in the same way as Mitchell's proton cycle [23] and the Na+ cycle [6]. It is tempting to suggest that the Cl--cycle helps N. pharaonis to survive in alkali environment, where application of the proton cycle could be difficult because of unfavorable DeltapH. In the framework of this conception, it is clear why N. pharaonis has Cl- pumping halorhodopsin, but does not have H+ pumping bacteriorhodopsin.
The authors thank Professor M. Engelhard for valuable advice and for donation of N. pharaonis strain. This work was supported by grants from the Russian Foundation for Basic Research (No. 96-04-50-938) and INTAS N 93-742-Extension.
REFERENCES
1.Tindall, B. J., Mills, A. A., and Grant, W. D.
(1980) J. Gen. Micr., 116, 257-260.
2.Bivin, D. B., and Stoeckenius, W. (1986) J. Gen.
Micr., 132, 2167-2177.
3.Lanyi, J. K., Duschl, A., Hatfield, G. W., May, K.,
and Oesterhelt, D. (1990) J. Biol. Chem., 265,
1253-1260.
4.Duschl, A., Lanyi, J. K., and Zimanyi, L. (1990)
J. Biol. Chem., 265, 1261-1267.
5.Scharf, B., Wittenberg, R., and Engelhard, M.
(1997) Biochemistry, 36, 4471-4479.
6.Skulachev, V. P. (1984) Trends Biochem.
Sci., 9, 483-485.
7.Broun, I. I. (1994) Biochemistry (Moscow),
59, 1779-1783 (Russ.).
8.Scharf, B., and Engelhard, M. (1994)
Biochemistry, 33, 6387-6393.
9.Duschl, A., Sawatzki, R., and Wagner, G. (1984)
EBEC, 3B, 633.
10.Hill, B., and Hill, A. E. (1973) J. Membr.
Biol., 12, 145-158.
11.Auffret, C. A., and Hanke, D. E. (1981)
Biochim. Biophys. Acta, 648, 186-191.
12.Geverscer, G. A., and Lee, S.-H. (1985)
Biochim. Biophys. Acta, 816, 415-417.
13.Gerencser, G. A., and Zelenza, B. (1993) Proc.
Natl. Acad. Sci. USA, 90, 7970-7974.
14.Gerencser, G. A. (1993) FEBS Lett.,
333, 137-140.
15.Gerencser, G. A., and Purushotham, K. R. (1996)
J. Bioenerg. Biomembr., 28, 459-469.
16.Gerencser, G. A. (1990) Biochim. Biophys.
Acta, 1030, 301-303.
17.Gradmann, D. (1984) in Chloride Transport
Coupling in Biological Membrane and Epithelia (Gerencser, G. A.,
ed.), pp. 13-61.
18.Tittor, J., Hansen, U.-P., and Gradmann, D.
(1983) J. Membr. Biol., 75, 129-139.
19.Ikeda, M., Schmid, R., and Oesterhelt, D. (1990)
Biochemistry, 29, 2057-2065.
20.Moritani, C., Ohhashi, T., Kadowaki, H., Tagaya,
M., Fukui, T., Lottspeich, F., Oesterhelt, D., and Ikeda, M. (1997)
Arch. Biochem. Biophys., 339, 115-124.
21.Ikeda, M., and Oesterhelt, D. (1990)
Biochemistry, 29, 2065-2070.
22.Ohhashi, T., Katsu, T., and Ikeda, M. (1992)
Biochim. Biophys. Acta, 1106, 165-170.
23.Mitchell, P. (1961) Nature, 191,
144-148.