Some Properties of Dissimilatory Nitrate Reductases Lacking Molybdenum and Molybdenum Cofactor
A. N. Antipov1, N. N. Lyalikova2, T. V. Khiznjak2, and N. P. L'vov1*
1A. N. Bach Institute of Biochemistry, Russian Academy of Sciences, Leninskii pr. 33, Moscow, 117071 Russia; fax: (095) 954-2732; E-mail: inbio@glas.apc.org2Institute of Microbiology, Russian Academy of Sciences, pr. 60-letiya Oktyabrya 7/2, Moscow, 117811 Russia
* To whom correspondence should be addressed.
Received November 19, 1998; Revision received January 27, 1999
Novel periplasmic and membrane-bound nitrate reductases lacking molybdenum and molybdenum cofactor were isolated from the vanadate-reducing bacterium Pseudomonas isachenkovii, and their properties were studied. Both enzymes have some unusual features, i.e., the individual subunits (130-kD subunit of the membrane-bound enzyme and monomeric 55-kD subunit of the periplasmic enzyme) possess their own nitrate reductase activity. In addition, both enzymes are highly thermostable, their temperature optimum being at 70-80°C, which is unexpectedly high for enzymes from mesophilic bacteria. Similarly to conventional molybdenum-containing nitrate reductases, these isolated enzymes are very sensitive to low concentrations of cyanide and azide. During anaerobic cell growth on medium with nitrate and vanadate, nitrate consumption is followed by a period of vanadate dissimilation, and this period is associated with some structural reorganizations of the nitrate reductases.
KEY WORDS: nitrate reductase, vanadium-containing enzymes, denitrification
Nitrate reductase (NR) is a key enzyme for nitrate assimilation in eukaryotes [1] and for nitrate assimilation or dissimilation in prokaryotes [2, 3]. The molybdenum dependence of NR was first mentioned in [4] and then confirmed by more than a half-century study of these enzymes [5]. All NRs of bacteria, algae, and plants characterized so far have molybdenum in a pterin molybdenum cofactor (Moco), the universal active site of these enzymes [6, 7]. Molybdenum in NR can be replaced on tungsten, but in this case the enzyme becomes inactive [8]. Vanadium does not replace molybdenum in NR of plants [9] and fungi [10] and unlike molybdenum and tungsten, is not included into molybdenum-free Moco-containing protein isolated from pea seeds [7]. Moreover, vanadium inhibits NR synthesis in plants [11]. All the results obtained so far indicate that molybdenum is unique as a component of the active site of NR. The only data pointing to a possible participation of vanadium in nitrate reduction were obtained by Indian scientists who showed that vanadium (but not molybdenum) stimulated NR activity of a tungsten-tolerant mutant of the cyanobacterium Nostoc muscorum [12, 13].
We supposed that vanadium-reducing facultative-anaerobic and facultative-chemolithotrophic Pseudomonas bacteria isolated by Lyalikova and Yurkova in the early 90s from vanadium-rich sources--sewage of a plant processing vanadium-containing slags (Pseudomonas vanadiumreductans) and ascidia, sea animals capable of accumulating vanadium from sea water and of concentrating it in vanadocytes, special blood chromophores (Pseudomonas isachenkovii) [14-16]--could be suitable for a search for vanadium-containing NR.
In the previous work [17] we reported on the isolation of novel membrane-bound and periplasmic NRs lacking molybdenum and Moco from P. isachenkovii cells. Also, vanadium was detected in the latter enzyme. The results of further study of these enzymes are discussed in this work.
MATERIALS AND METHODS
In this study we used the following reagents: glycine, acrylamide, ammonium persulfate, TEMED, and triphenyltetrazolium chloride from Sigma (USA); methyl viologen and N-1-naphthylethylenediamine from Serva (Germany); Tris and sodium dithionite from Merck (Germany); bis-acrylamide, NaN3, and KCN from Fluka (Switzerland); yeast extract from Difco (USA). All other reagents were of extra pure grade produced in Russia.
Preparation of biomass. P. isachenkovii cells were grown under anaerobic conditions at 30°C for 4-8 days in a 10-liter bottle on modified Postgate medium containing 0.25 g/liter KH2PO4, 0.5 g/liter NH4Cl, 1.0 g/liter NaNO3, 0.5 g/liter NaVO3·2H2O, 1.0 g/liter sodium lactate, and 0.2 g/liter yeast extract. The medium also contained the necessary microelements and trace amounts of MgSO4·7H2O and FeSO4·7H2O. Biomass was harvested after the medium became blue, which indicated that all nitrate had been consumed and the reduction of V5+ to V4+ had been completed.
Preparation of cell extract. The cells were collected by centrifugation at 4,500g for 45 min, washed with 25 mM sodium phosphate buffer (pH 6.8), and disintegrated at 250-280 atm using a French press. To study NR activity as a function of cell growth, the cells were ultrasonicated using a UZDN-1 disintegrator (22 kHz, 3 min, 5°C). The extract was centrifuged at 15,000g for 30 min. The supernatant was used as a source of periplasmic NR. The pellet was suspended in 25 mM sodium phosphate buffer containing 5% Triton X-100 for 12 h at 4°C using a magnetic stirrer. Then the suspension was centrifuged at 15,000g for 30 min, and the supernatant was used as a source of membrane-bound NR. Homogeneous nitrate reductase preparations were obtained as described in our previous work [17].
Assay of NR activity. Reduced methyl viologen was used as a donor of electrons when assaying NR activity. The reaction mixture contained 100 mM sodium phosphate buffer (pH 6.8), 0.7 mM methyl viologen, 10 mM NaNO3, 1.15 mM sodium dithionite, and 0.01-0.1 ml of the studied preparation. The reaction was initiated by addition of dithionite, performed at 70°C, and stopped by addition of 500 µl of 0.6% sulfanilic acid in 20% HCl and 500 µl of 2 mM N-1-naphthylethylenediamine. Absorbance at 548 nm was measured after 15 min necessary for color development.
To determine nitrate reductase activity in polyacrylamide gel, reaction mixture containing 0.2 M sodium phosphate buffer (pH 6.8), 20 mM KNO3, 1 mM methyl viologen, and 5 mM sodium dithionite was used. The gel was immersed into this mixture and incubated at 70°C until transparent bands appeared against the blue background of the gel because of oxidation of methyl viologen by nitrate reductase. Then the gel was placed in 0.05% solution of triphenyltetrazolium chloride to fix the staining. Acetic acid (5%) was used for the final fixation of the gel.
Electrophoresis in polyacrylamide gel. Native electrophoresis on 11 × 11-cm plates was performed through a gradient polyacrylamide gel (5-15%) in Tris-HCl buffer (pH 8.8) in the system of Davis [18]. Current was 40 mA in the separating mode. The following standard set of proteins from Serva was used: catalase (240 kD), aldolase (147 kD), BSA (67 kD), ovalbumin (45 kD), and carbonic anhydrase (29 kD). The proteins were stained with silver according to Nesterenko [19].
Protein concentration was determined according to Bradford [20] using bovine serum albumin as a standard.
Nitrate was determined according to Cataldo et al. [21].
Concentration of V4+ was determined titrimetrically [22].
RESULTS AND DISCUSSION
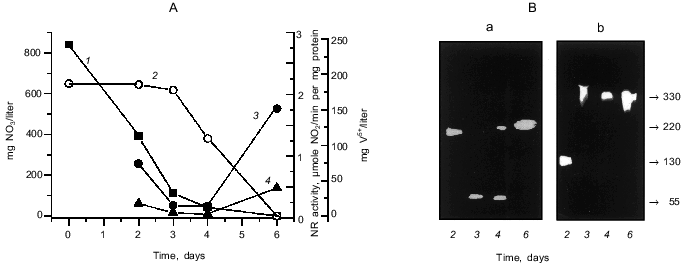
The results of determination of nitrate and vanadate (V5+) in the medium during anaerobic growth of P. isachenkovii are presented in Fig. 1A. Bacterial cells intensively consumed nitrate during denitrification, usually during the first three days of growth. The second substrate, vanadate, is not dissimilated until nitrate concentration decreases to its lowest value. Calculations performed earlier give an explanation for this [23]. The dynamics of the activity of the membrane-bound and periplasmic NRs during cell culture growth (Fig. 1A) to some extent reflect cell transfer from denitrification to reduction of vanadate: activity decreases together with the disappearance of nitrates from the medium and then, after some time lag perhaps related to “rearrangement” of the enzyme because of the change of the substrate, begins to increase for both enzymes.
As we demonstrated earlier [17], the membrane-bound NR from P. isachenkovii has molecular mass 330 kD (subunits 130 and 67 kD) and the periplasmic NR has molecular mass 220 kD and consists of 55-kD monomers. According to the results of determination of NR activity in the gel (Fig. 1B), denitrification involving the membrane-bound NR is first performed by a large subunit of the enzyme (130 kD) which possesses its own NR activity, as we showed in our previous work [17]. As the reduction of vanadate in the cells begins, an oligomeric enzyme complex of the membrane-bound NR with molecular mass 330 kD is detected. The structure of periplasmic NR also changes during cell transfer from denitrification to vanadate reduction (Fig. 1B): denitrification is first performed by an oligomeric enzyme with molecular mass 220 kD, and after nitrate consumption in the medium the enzyme is detected in the form of a monomer with molecular mass 55 kD, and on the increase of vanadate-reducing activity of the cells it is detected again as an oligomer with molecular mass 220 kD.Fig. 1. Reduction of nitrate and vanadate and nitrate reductase activity during anaerobic growth of P. isachenkovii. A) Dynamics of reduction of nitrate (1) and vanadate (2) and activities of the membrane-bound (3) and periplasmic (4) NRs. B) Change of molecular masses of periplasmic (a) and membrane-bound NRs (b) during culture growth. Molecular masses (on the right, in kD) were determined by native gradient (5-15%) electrophoresis in polyacrylamide gel. The procedure of NR determination in the gel is described in “Materials and Methods”.
We suppose that changes in the activity and molecular structure of the enzymes described above point to the participation of NR in reduction of not only nitrate, but also vanadate.
Let us discuss some properties of NR isolated from P. isachenkovii cells. First, we would like to note the above-mentioned capacity of individual subunits of the membrane-bound (130 kD) and periplasmic (55 kD) NRs to exhibit their own NR activity, as already described in our first work [17]. The high activities of these subunits allows us to detect them in polyacrylamide gel (Fig. 1B). The activity of individual subunits seems to have physiological importance for the cell because subunits rather than oligomeric complexes can be used by the cell for reduction of nitrate and possibly vanadate. Such activity of the enzyme subunits was not detected earlier for any NR described in the literature. Only in several works [3] an active site of NR, Moco, was found to be localized on the large subunits of dissimilatory NR, although the individual catalytic activity of subunits was not demonstrated in these studies.
Judging from some of their properties (molecular mass, temperature optimum, and others), the novel NRs we have isolated from anaerobically growing P. isachenkovii cells seem to be synthesized also in aerobically growing cells, but in markedly lower amounts (Fig. 2).
The nitrate reductases we isolated can be synthesized in the presence of high concentrations of ammonium in the medium; in this respect they differ from the molybdenum-containing dissimilatory NR.Fig. 2. Determination of nitrate reductase activity of extracts of P. isachenkovii cells grown under anaerobic (membrane bound (1) and periplasmic (2) NRs) and aerobic (membrane-bound (3) and periplasmic (4) NRs) conditions. NR samples from aerobic cells contained 50 µg of protein and that from anaerobic cells contained 5 µg of protein.
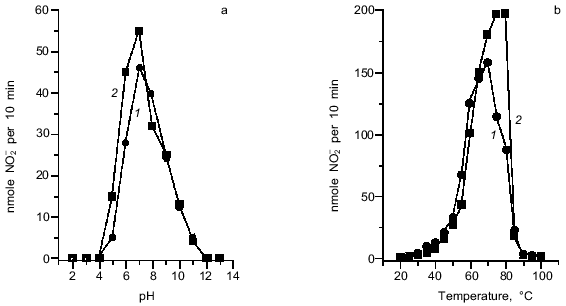
Activities of both NRs are maximal at pH 6.8-7.0, although rather high activity was detected in the wider range of pH from 6.0 to 8.0 (Fig. 3a). The studied NRs have temperature optimum at 70-80°C (Fig. 3b); this is unexpectedly high for enzymes from mesophilic bacteria. The activity of both enzymes remained unchanged at 50°C for 2 h, whereas incubation at 70°C for 40 min resulted in the loss of 80% of the NR activity. High thermal stability is also typical of vanadium-containing haloperoxidases [24-26].
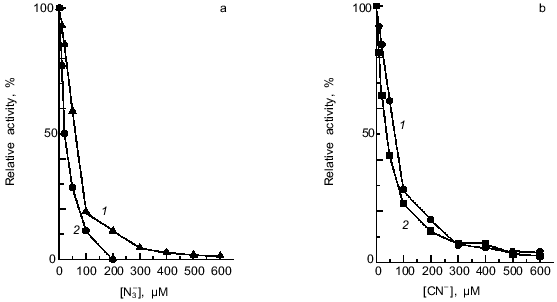
The isolated NRs were inhibited by low concentrations of cyanide or azide; this feature is typical of the conventional molybdenum-containing dissimilatory NRs. I0.5 was 70 and 60 µM for the membrane-bound and 40 and 20 µM for the periplasmic NR, respectively (Fig. 4). Inhibition by cyanide and azide suggests the presence of a metal atom in the active site.Fig. 3. pH (a) and temperature (b) optima for nitrate reductase activities: 1) membrane-bound NR; 2) periplasmic NR. Buffers used in (a): 0.2 M glycine-HCl, pH 2-3; 0.2 M Na-AcOH, pH 4-5; 0.2 M sodium phosphate, pH 6-8; 0.2 M glycine-NaOH, pH 9-10; 0.2 M Na2HPO4-NaOH, pH 11; 0.2 M KCl-NaOH, pH 12-13. In (b) the standard reaction mixture for determination of nitrate reductase activity was used. In both (a) and (b) data for homogeneous preparations of both NRs are shown.
Thus, two novel NRs lacking molybdenum and Moco (one of them containing vanadium) were isolated from P. isachenkovii, and this resembles the situation in the study of nitrogenase which was done in the early 80s. Then, first a vanadium-containing nitrogenase and subsequently an iron-containing nitrogenase lacking molybdenum and vanadium were discovered [27-29]. Along with the alternative nitrogenase, vanadium was also detected in some haloperoxidases catalyzing oxidation of halogen ions by hydrogen peroxide (bromo-, chloro-, and iodoperoxidases), as mentioned above. Several haloperoxidases have structures similar to that of the periplasmic NR isolated by us [24]. Except for the alternative nitrogenase and the haloperoxidases, we do not know of any other vanadium-containing enzymes being reported [30, 31].Fig. 4. Effect of azide (a) and cyanide (b) on the activities of nitrate reductases: 1) membrane-bound NR; 2) periplasmic NR. Homogeneous NR preparations of both types were used in the experiments.
The isolation of a novel NR lacking molybdenum and Moco from vanadium-reducing bacteria raises some questions: are these alternative enzymes widespread, and under what conditions and in which bacteria are they synthesized? Concerning the vanadium-containing NR, it is necessary first to remember that the content of vanadium in the biosphere is 20 times greater than that of molybdenum [32]; perhaps in regions with decreased molybdenum content in the soil, molybdenum-deficient plants can synthesize the alternative vanadium-containing NR. The appearance of the alternative NR is especially possible in bacteria growing at high concentrations of toxic heavy metals, when synthesis of the normal molybdenum-containing NR is suppressed. This seems to occur in cells of the above-mentioned tungsten-tolerant mutant of the cyanobacterium Nostoc muscorum [12, 13] and an E. coli mutant tolerant to high concentrations of tellurite and selenate [33]. Ivanova's studies on the nitrate reductase activity of plant peroxidases published in the 70s and 80s should be also mentioned [34-37]. These enzymes use superoxide radicals for reduction of nitrates and can to some extent replace the normal NR in plant growth under stress conditions. These works as well as our isolation of novel nitrate reductases demonstrate that alternative pathways of nitrate reduction could be used by living organisms.
This work was financially supported by the Russian Foundation for Basic Research (grant 98-04-48348).
REFERENCES
1.Solomonson, L. P., and Barber, M. J. (1990) Ann.
Rev. Plant. Physiol. Plant Mol. Biol., 41, 225-253.
2.Hoff, T., Stumman, B. M., and Henningen, K. W.
(1992) Physiol. Plantarum, 84, 616-624.
3.Zumft, W. G. (1997) Microbiol. Mol. Biol.
Rev., 61, 533-616.
4.Wolfe, M. (1954) Ann. Bot., 18,
299-308.
5.L'vov, N. P. (1989) Molybdenum in Nitrogen
Assimilation in Plants and Microorganisms. 43rd A. N. Bach Memorial
Lecture [in Russian], Nauka, Moscow.
6.Rajagopalan, K. V. (1984) in Molybdenum Enzymes,
Cofactors and Model Systems, ACS Symp. Ser. (Stiefel, E. I.,
Coucouvanis, D., and Newton, W. E., eds.) ACS, Washington, DC, pp.
34-49.
7.Kildibekov, N. A., Omarov, R. T., Antipov, A. N.,
Shvetsov, A. A., Mironov, E. A., and L'vov, N. P. (1996) Plant
Physiol. Biochem., 34, 677-682.
8.Deng, M., Moureaux, T., and Caboche, M. (1989)
Plant. Physiol., 91, 304-309.
9.Notton, B. A., and Hewitt, E. J. (1972) Biochim.
Biophys. Acta, 275, 355-357.
10.Ninneman, H. (1990) J. Plant Physiol.,
137, 677-682.
11.Somasundaram, R., Muthuchelian, K., and
Murugesan, S. (1994) J. Environ. Biol., 15, 41-48.
12.Singh, H. M., Chakravarty, D., Srinivasa Rao, K.,
and Singh, A. K. (1993) J. Basic Microbiol., 33,
201-205.
13.Singh, S., Chakravarty, D., and Singh, H. M.
(1993) Biochem. Mol. Biol. Int., 29, 1083-1093.
14.Lyalikova, N. N., and Yurkova, N. A. (1989) in
Proc. VI Int. Trace Element Symp., Vol. 1, Jena, pp.
74-78.
15.Yurkova, N. A., and Lyalikova, N. N. (1990)
Mikrobiologiya, 59, 968-975.
16.Lyalikova, N. N., and Yurkova, N. A. (1992)
Geomicrob. J., 10, 15-26.
17.Antipov, A. N., Lyalikova, N. N., Khizhnjak, T.
V., and L'vov, N. P. (1998) FEBS Lett., 441, 257-260.
18.Davis, B. J. (1965) Ann. N. Y. Acad. Sci.,
121, 404-407.
19.Nesterenko, M. V. (1994) Biochem. Biophys.
Meth., 28, 239-242.
20.Bradford, M. A. (1976) Anal. Biochem.,
72, 248-254.
21.Cataldo, D. A., Haroon, M., Shrader, L. E., and
Youngs, V. L. (1975) Commun. Soil Sci. Plant Anal.,
6, 71-80.
22.Kitaigorodski, I. I., and Frolov, V. K. (1960)
Zavodsk. Labor., 26, 418-422.
23.Yurkova, N. A., Savel'eva, N. D., and Lyalikova,
N. N. (1993) Mikrobiologiya, 62, 597-603.
24.Plat, H., Krenn, B. E., and Wever, R. (1987)
Biochem. J., 248, 277-279.
25.Wever, R., Plat, H., and de Boer, E. (1985)
Biochim. Biophys. Acta, 830, 181-186.
26.Van Schijndel, J. W., and Barnett, P. (1994)
Eur. J. Biochem., 225, 151-157.
27.Robson, R. L., Eady, R. R., Richardson, T. H.,
Miller, R. W., Hawkins, M., and Postgate, J. R. (1986) Nature,
322, 288-390.
28.Bishop, P. E., and Premakumar, R. (1988) in
Biological Nitrogen Fixation (Stacey, G., Burris, R. H., and
Evans, H. J., eds.) Chapman & Hall, N. Y.
29.Chisnell, J. R., Premakumar, R., and Bishop, P.
E. (1988) J. Bacteriol., 170, 27-33.
30.Smith, M. J. (1989) Experimentia,
45, 452-457.
31.Rehder, D. (1992) Biometals, 5,
3-12.
32.Kholodov, V. N. (1968) Vanadium: Geology,
Mineralogy, and Genetic Types of Deposits in Sedimentary Rocks [in
Russian], Nauka, Moscow.
33.Avazeri, C., Turner, R. J., Pommier, J., Weiner,
J. H., Giordano, G., and Vermeglio, A. (1997) Microbiology,
143, 1181-1189.
34.Peive, Ya. V., Ivanova, N. N., Ovcharenko, G. A.,
and Shirinskaya, M. G. (1975) Sov. Plant Physiol., 22,
527-535.
35.Ivanova, N. N., and Drobysheva, N. I. (1976)
Sov. Plant Physiol., 23, 1141-1148.
36.Ivanova, N. N., Ovcharenko, G. A., Khudyakova, E.
M., and Roshchupkina, T. G. (1979) Sov. Plant Physiol.,
26, 302-308.
37.Ivanova, N. N., and Drobysheva, N. I. (1985)
Sov. Plant Physiol., 32, 488-493.