Photoelectric Responses of Oxygen-Evolving Complexes of Photosystem II
M. D. Mamedov1*, O. E. Beshta2, K. N. Gurovskaya1, A. A. Mamedova1, K. D. Neverov3, V. D. Samuilov2, and A. Yu. Semenov1
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: mahir@phtbio.genebee.msu.su2School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3807
3Bach Institute of Biochemistry, Russian Academy of Sciences, Leninskii pr. 33, Moscow, 117071 Russia
* To whom correspondence should be addressed.
Received December 7, 1998; Revision received February 8, 1999
The generation of a transmembrane electric potential difference induced by a series of laser flashes was studied by the direct electrometrical method in proteoliposomes containing oxygen-evolving particles of photosystem II. In addition to the fast stage of generation of the membrane potential, which is due to electron transfer from the redox active tyrosine residue Tyr-161 (YZ) to the primary quinone acceptor QA, electrogenic stages corresponding to the S1 --> S2 (tau = 30 µsec), S2 --> S3 (tau = 240 µsec), and S3 --> S4 --> S0 (tau = 6.2 msec) transitions of the oxygen-evolving complex (OEC) were observed. The amplitudes of the photoelectric responses show that the contribution of the OEC to the overall electrogenicity is small. The parameters of the electrogenic reactions of the OEC as measured in photosystem II preparations containing the peripheral proteins of 23 and 17 kD were similar to those of photosystem II preparations devoid of these peptides. It is concluded that neither the 23- nor the 17-kD proteins are involved in the electrogenic reactions of the OEC.
KEY WORDS: photosystem II, oxygen-evolving complex, proteoliposomes, S states, electrogenicity
Photosystem (PS) II of higher plants, algae, and cyanobacteria mediates photoinduced oxidation of water and reduction of plastoquinone. An electron from the singlet excited state of the primary electron donor of PS II (P680) is transported to pheophytin. Then, the reduced pheophytin transfers an electron to the primary quinone acceptor (QA) and further to the secondary quinone acceptor (QB). The cation-radical P680+ is reduced by the redox active tyrosine residue Tyr-161 of the D1 subunit of PS II (YZ) [1, 2]. Oxidized YZ is then reduced by the manganese cluster of the oxygen-evolving complex (OEC) [3]. This complex contains four manganese atoms and cofactor X. Most probably, the cofactor X is a histidine residue which is in redox equilibrium with YZ. During sequential transfer of four electrons from two molecules H2O to photooxidized P680+, the redox state (S) of the OEC goes through four transitions from S0 to S4, where subscripts 0-4 represent the value of the positive charge accumulated in the OEC. In dark-adapted preparations, the OEC is dominantly in the state S1 (S1/S0 = 75-80%). During transitions S0 --> S1 and S1 --> S2, the manganese cluster sequentially loses two electrons. Transition S2 --> S3 is probably accompanied by oxidation of component X. Transition S3 --> S4 is accompanied by oxidation of YZ. State S4 is unstable; it releases O2 and reverts spontaneously to S0 [4].
It was shown using the electrometrical method that electron transfer from YZ to QA [5, 6] and protonation of reduced plastoquinone QB in PS II [7, 8] are accompanied by the generation of a transmembrane electric potential difference (DeltaPsi). Electrochromic absorption changes at 522 nm in pea thylakoids [9] showed that the H+ uptake associated with reduction of non-heme iron at the acceptor side of PS II is also electrogenic. The electrogenicity associated with the functioning of the OEC in PS II has been studied in spinach chloroplasts by monitoring chlorophyll electroluminescence [10], in thylakoid membranes using electrochromic absorption spectroscopy at 522 nm, and in pea PS II complexes containing no 23- and 17-kD peripheral proteins of the OEC by direct electrometry [11].
The goal of this work was to study electrogenic reactions associated with the functioning of the OEC in spinach PS II particles containing 23- and 17-kD peripheral proteins using the method of direct electrometry.
MATERIALS AND METHODS
The following reagents were used: azolectin (soybean phosphatidylcholine type II-S), sodium cholate, octyl glucoside, potassium ferricyanide, glycine betaine, phenyl-p-benzoquinone, and CaCl2 from Sigma (USA); MES, Tricine, and Tris from Serva (Germany); and a number of additionally recrystallized special purity grade products of domestic suppliers.
Photosystem II particles with an active water oxidation system were isolated from spinach as described in [12]. These particles contain peripheral OEC proteins with molecular weights of 23 and 17 kD. The rate of O2 evolution was measured polarographically using a closed-type Clark electrode. Photosystem II particles with inactive OEC were prepared by incubation of a suspension of active particles with a chlorophyll concentration of 5 µM for 10 min in a medium containing 0.9 M Tris-HCl (pH 9). Before measurements, the suspension pH was adjusted to 6.5. The rate of O2 evolution in these preparations did not exceed 5-10% of the initial value.
Proteoliposomes were prepared as follows: an azolectin suspension (40 mg/ml) in 25 mM Tricine-NaOH buffer solution (pH 7.5) containing 2% sodium cholate and 1 M glycine betaine was sonicated (22 kHz, 40 mA) in a UZDN-2T ultrasonic disintegrator until a transparent solution was obtained. The resulting solution was mixed with PS II particles at a lipid/protein ratio of 30 (w/w). The resulting mixture was applied to a chromatographic column (5 × 20 cm) filled with Sephadex G-50 equilibrated with a buffer solution containing 20 mM Tricine-NaOH and 10 mM CaCl2 (pH 7.5). The proteoliposomes were eluted with the same buffer solution. All operations of the proteoliposome preparation were carried out at 4°C.
Transmembrane potential generation was detected by the direct electrometrical method as described in [13]. The incubation medium contained 10 mM CaCl2, 1 M glycine betaine, and 20 mM MES-NaOH (pH 6.5). Light pulses of saturated intensity (wavelength, 530 nm; FWHM, 15 nsec) were generated by a frequency-doubled Q-switched YG-481 Nd:YAG laser (Quantel, France). The time interval between successive light flashes was 1 sec. Redox titration of the photoelectric response was performed as described in [8].
RESULTS AND DISCUSSION
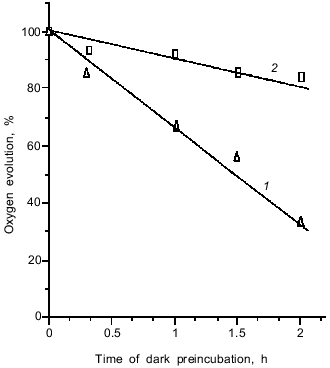
The oxygen-evolving complex of PS II is the most labile component of the electron-transport chain. It is seen from Fig. 1 that glycine betaine, an effective osmoregulator of halophilic bacteria, is able to stabilize the OEC activity. This ability is probably due to prevention of manganese loss from the water-oxidizing complex of PS II [14].
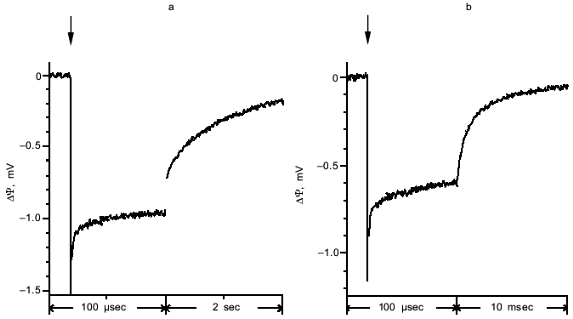
A typical photoelectric response induced in proteoliposomes containing PS II particles by a single laser flash is shown in Fig. 2a. The proteoliposomes were associated with a measuring collodion membrane impregnated with a solution of phospholipids in n-decane. As shown in the preceding work [8], association of PS II vesicles with the collodion membrane is accompanied by extraction of the secondary quinone acceptor QB. As a result, no electrogenic reactions of QB reduction were observed in the system studied in the present work. The light pulse induced the generation of a transmembrane potential (minus inside) on the proteoliposome membrane, the kinetics of this process being faster than the time resolution of the measuring system. As shown in earlier works [8, 11], the kinetic phase of transmembrane potential generation which attains its maximum within a time interval shorter than 0.1 µsec is due to charge separation between the tyrosine YZ and the plastoquinone QA. This conclusion was supported by the fact that the amplitude of the photoelectric response declines along with equilibrium chemical reduction of QA, which brings the reaction centers of PS II into a photochemically inactive state (not shown). The midpoint redox potential value E0 of the photoelectric response (-80 mV) corresponds to the E0 value of QA in PS II particles with a functionally active OEC [15].Fig. 1. Effect of glycine betaine on the rate of oxygen evolution by proteoliposomes containing PS II particles: 1) control; 2) in the presence of 1 M glycine betaine. Chlorophyll concentration, 8 µg/ml. The incubation medium contained 10 mM CaCl2, 100 µM phenyl-p-benzoquinone, and 20 mM MES-NaOH (pH 6.5). 100% corresponds to 950 µmoles O2/h per mg chlorophyll.
Computer processing showed that the decay of the photoelectric response can be approximated by four exponential components with characteristic times (tau) of 1 sec, 13 msec, 20 µsec, and 1 µsec. The relative amplitudes of the components are 50, 26, 12, and 12%, respectively. The component with tau = 13 msec is probably due to charge recombination between reduced QA- and oxidized YZ (YZox) in PS II particles with inactive OEC [16]. Fast kinetic phases of the photoelectric response decay (tau = 20 and 1 µsec) were observed only if the laser flash energy was higher than 10 mJ. The component with tau = 1 sec is probably due to relaxation of a charge-transfer state in PS II particles with an active OEC [15]. This conclusion is supported by the fact that this slow component is absent in the photoelectric responses of proteoliposomes containing inactivated OEC (Fig. 2b). This suggests that the O2-evolving activity is conserved only in 50% of the PS II particles incorporated into liposomes.Fig. 2. Photoelectric responses of proteoliposomes containing PS II particles with active (a) and inactive (b) OEC. The incubation medium contained 10 mM CaCl2, 1 M glycine betaine, 100 µM phenyl-p-benzoquinone, and 20 mM MES-NaOH (pH 6.5). Here and in other figures a vertical arrow shows the time of the laser flash. Note that the time scales in (a) and (b) are different.
According to the sign of the photoelectric response (minus inside proteoliposome), the OEC is located near the outer surface of the proteoliposome membrane. Therefore, proteoliposomes with PS II particles provide an appropriate model for studying electrogenic reactions of the OEC because the activity of the complex can be stabilized with glycine betaine, an agent which can not permeate through the proteoliposome membrane. A lack of an effect of potassium ferricyanide, an impermeable electron acceptor, on the amplitude of the photoelectric response can be regarded as evidence for unidirectional orientation of the PS II complexes in the phospholipid vesicles (not shown). Similar unidirectional orientation was recently shown to be typical of PS II complexes isolated from cyanobacteria [6, 8].
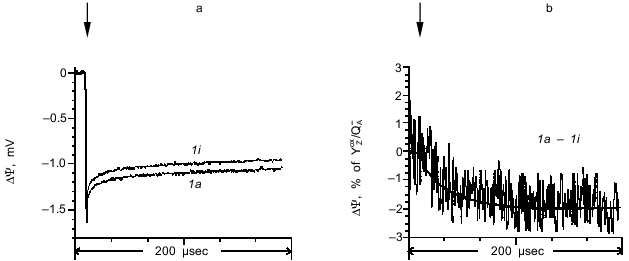
To reveal the electrogenic phases corresponding to transitions between the S states, photoelectric responses induced by sequential laser flashes were compared to each other. The photoelectric responses induced by the first laser flash in dark-adapted proteoliposomes containing active and inactive OEC are shown in Fig. 3a (curves 1a and 1i, respectively). The difference between curves 1a and 1i (Fig. 3b) shows that the maximum amplitude of the electrogenic stage corresponding to the OEC transition from S1 to S2 is 1.7% of the amplitude of the fast phase corresponding to the YZoxQA- dipole formation, its characteristic time being 30 µsec.
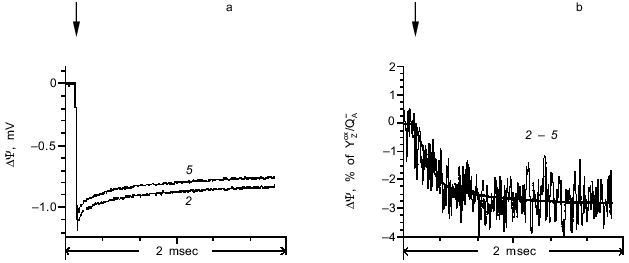
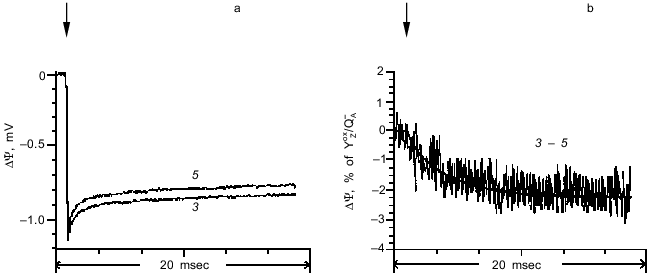
The photoelectric responses induced by the second (transition S2 --> S3) and fifth laser flashes are shown in Fig. 4a. The difference between the responses induced by the second and fifth laser flashes is shown in Fig. 4b. Like the first flash, the fifth flash induces the S1 --> S2 transition, but without the contribution from non-heme iron [11]. Although the amplitude of the photoelectric response induced by the fifth flash was less than in response to the first flash, the kinetic characteristics of the two signals were virtually indistinguishable. Analysis of the photoelectric responses showed that the kinetic phase corresponding to the transition S2 --> S3 has a characteristic time of 240 µsec, whereas its amplitude is 2.8% of the amplitude of the fast phase corresponding to the YZoxQA- dipole formation.Fig. 3. a) Photoelectric responses induced by the first laser flash in dark-adapted proteoliposome preparations containing PS II particles with active (curve 1a) and inactive (curve 1i) OEC. b) Difference between curves 1a and 1i. The curves were normalized to the fast phase of the photoelectric response corresponding to the YZoxQA- dipole formation in the proteoliposomes with active OEC.
The photoelectric responses induced by the third (transition S3 --> S4 --> S0) and fifth laser flashes are shown in Fig. 5a, and the difference between the signals is shown in Fig. 5b. This transition has a characteristic time of 6.2 msec, and its amplitude is 2% of the amplitude of the phase corresponding to the YZoxQA- dipole formation.Fig. 4. a) Photoelectric responses induced by the second (curve 2) and fifth (curve 5) laser flashes in dark-adapted proteoliposome preparations containing PS II particles. b) Difference between curves 2 and 5. The curves were normalized to the fast phase of the photoelectric response induced by the second flash.
It should be noted that the kinetic characteristics of the phases of the photoelectric response observed in these experiments are fully consistent with the results of measurements of light-induced absorption changes at 360 nm which represent the S-state transitions in the OEC [11]. As the time interval between the laser flashes is increased, there was a decrease in the amplitude of the phases corresponding to transitions S2 --> S3 and S3 --> S4 --> S0. This decrease can be attributed to relaxation of states S2 and S3 into S1 in the dark [17].Fig. 5. a) Photoelectric responses induced by the third (curve 3) and fifth (curve 5) laser flashes in dark-adapted proteoliposome preparations containing PS II particles. b) Difference between curves 3 and 5. The curves were normalized to the fast phase of the photoelectric response induced by the third flash.
Neither the 3-D structure of the OEC nor the relative distances between the manganese cluster and redox cofactors X and YZ is presently known. Indirect data obtained by EPR spectroscopy suggest that the manganese cluster is at a distance of 8-20 Å from YZ [18-20]. On the other hand, according to electron-nuclear double resonance (ENDOR) spectroscopy, the distance between these centers is ~4.5 Å [21, 22]. The results of the present work show that the relative amplitudes of the electrogenic reactions associated with electron transfer from the manganese cluster to YZ (S1 --> S2 transition induced by the first flash), oxidation of component X by the tyrosine residue YZox (S2 --> S3 transition induced by the second flash) [23], and the S3 --> S4 --> S0 transition are ~3.4, 5.6, and 4% of the phase corresponding to the YZoxQA- dipole formation, respectively. Taking into account the small relative amplitudes of all photoelectric signals associated with OEC functions, it can be suggested that either the projections of the distances between YZ, manganese cluster, and component X onto the perpendicular to the membrane plane are small or the dielectric constant (epsilon) value at this site of PS II are much higher than the epsilon value at the membrane site between YZ and QA. According to the three-phase model of membrane proteins [24], the epsilon value at the sites of location of peripheral redox centers spatially remote from the hydrophobic core of membrane proteins is significantly increased. Therefore, the second interpretation seems more realistic in the context of this theory.
Thus, the S1 --> S2 (tau = 30 µsec), S2 --> S3 (tau = 240 µsec), and S3 --> S4 --> S0 (tau = 6.2 msec) transitions of the PS II OEC are electrogenic. The parameters of the electrogenic reactions of the OEC as measured in PS II preparations containing the 23- and 17-kD peripheral proteins were similar to the electrogenic reactions of PS II preparations devoid of these peptides [11]. Therefore, it can be concluded that neither the 23- nor the 17-kD proteins are involved in the electrogenic reactions of the OEC of PS II.
This study was supported by the Russian Foundation for Basic Research (project Nos. 97-04-50179 and 98-04-48226), the International Scientific-Engineering Foundation (project No. 866), INTAS (project No. 93-2852), and the President of the Russian Federation Foundation for Young Doctors of Sciences (project No. 96-15-97043).
REFERENCES
1.Debus, R. J., Boar, B. A., Sithole, I., Babcock, G.
T., and McIntosh, L. (1988) Biochemistry, 27,
9071-9074.
2.Metz, J. G., Nixon, P. J., Rogner, M., Brudvig, G.
W., and Diner, B. A. (1989) Biochemistry, 28,
6960-6969.
3.Dekker, J. P., Van Gorkom, H. J., Wensink, J., and
Ouwehand, L. (1984) Biochim. Biophys. Acta, 767, 1-9.
4.Ahlbrunk, R., Haumann, M., Cherepanov, D.,
Bogershausen, O., Mulkidjanian, A., and Junge, W. (1998)
Biochemistry, 37, 1131-1142.
5.Pokorny, A., Wulf, K., and Trissl, H.-W. (1994)
Biochim. Biophys. Acta, 1184, 65-70.
6.Mamedov, M. D., Lovyagina, E. R., Verkhovskii, M.
I., Semenov, A. Yu., Cherepanov, D. A., and Shinkarev, V. P. (1995)
Biochemistry (Moscow), 59, 327-341 (Russ.).
7.Hook, F., and Brzezinski, P. (1994) Biophys.
J., 66, 2066-2072.
8.Mamedov, M. D., Beshta, O. E., Samuilov, V. D., and
Semenov, A. Yu. (1994) FEBS Lett., 350, 96-98.
9.Haumann, M., Hundelt, M., Drevenstedt, W., and
Junge, W. (1995) in Photosynthesis: From Light to Biosphere
(Mathis, P., ed.) Kluwer, Dordrecht (The Netherlands), pp. 333-336.
10.Voss, M. H., Van Gorkom, H. J., and Van Leeuwen,
P. J. (1991) Biochim. Biophys. Acta, 1056, 27-39.
11.Haumann, M., Mulkidjanian, A., and Junge, W.
(1997) Biochemistry, 36, 9304-9315.
12.Ghanotakis, D. F., Demetriou, D. M., and Yocum,
C. F. (1987) Biochim. Biophys. Acta, 891, 15-26.
13.Drachev, L. A., Semenov, A. Yu., Skulachev, V.
P., Smirnova, I. A., Chamorovsky, S. K., Kononenko, A. A., Rubin, A.
B., and Uspenskaya, N. Ya. (1981) Eur. J. Biochem., 117,
483-489.
14.Mohanty, P., Hayashi, H., Papageorgiou, G. C.,
and Murata, N. (1993) Biochim. Biophys. Acta, 1144,
92-96.
15.Rutherford, A. W., and Boussac, A. (1992) in
Research in Photosynthesis. Proc. 9th Int. Congr. on
Photosynthesis (Murata, N., ed.) Vol. 2, Kluwer, Dordrecht (The
Netherlands), pp. 21-27.
16.Bogershausen, O., and Junge, W. (1995)
Biochim. Biophys. Acta, 1230, 177-185.
17.Van Leewen, P. J., Heiman, C., Gast, P., Dekker,
J. P., and van Gorkom, H. J. (1993) Photosynth. Res., 38,
169-176.
18.Hogansson, C. W., and Babcock, G. T. (1989)
Biochemistry, 28, 1448-1454.
19.Swensson, B., Vass, I., and Styring, S. (1991)
Z. Naturforsch., 46, 765-776.
20.Un, S., Brill, T. M., and Zimmermann, J. L.
(1994) Proc. Natl. Acad. Sci. USA, 91, 5262-5266.
21.Gilchrist, M. L., Ball, J. A., Randall, D. W.,
and Britt, R. D. (1995) Proc. Natl. Acad. Sci. USA, 92,
9545-9549.
22.Britt, R. D. (1996) in Oxygenic
Photosynthesis: The Light Reactions (Ort, D., and Yocum, C. F.,
eds.) Kluwer, Dordrecht (The Netherlands), pp. 137-164.
23.Boussac, A., Zimmermann, J. L., Rutherford, A.
W., and Lavergne, J. (1990) Nature, 347, 303-306.
24.Cherepanov, D. A., and Krishtalik, L. I. (1990)
Bioelectrochem. Bioenerg., 24, 113-127.