Age-Related Changes in Antioxidant and Glutathione S-Transferase Enzyme Activities in the Asian Clam
J. Vranković
University of Belgrade, Institute for Biological Research “Siniša Stanković”, Department of Ecology, 11060 Belgrade, Serbia; fax: + (381) 11-2761-433; E-mail: jeca.s@ibiss.bg.ac.rs
Received September 2, 2015; Revision received September 30, 2015
Aging is accompanied by increased production of free oxygen radicals and impairment of normal cellular functions. The aim of this work was to provide preliminary data on age-related differences in the activities of antioxidant enzymes and phase II biotransformation enzyme glutathione S-transferase (GST) in a wild population of the Asian clam Corbicula fluminea. The antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), and GST were assessed in visceral mass of four age classes (0+-, 1+-, 2+-, and 3+-year-old) of C. fluminea clams. Age-related changes were seen in antioxidant enzyme status: levels of total SOD (totSOD) (P < 0.05), MnSOD, and CuZnSOD (P < 0.05) activities increased progressively during aging from younger to older clams. Changes in CAT and GR activities with advancing age were found, the levels being the highest in age class II, then being lower in age classes III and IV (P < 0.05). Activities of GPX and GST were lower in the senescent individuals (2+- and 3+-year-old clams) compared with young individuals (0+- and 1+-year-old clams). Overall, the decline of glutathione-dependent enzyme activities, coupled with higher and lower activities of totSOD and CAT, respectively, as the individual grows older, may render the older animals more susceptible to oxidative stress. Data reported here are not intended to be exhaustive since they concern only age/size structure of the population at one locality, so more detailed studies on both the developmental stages and levels of antioxidant enzymes of this new alien species in Serbian rivers are required.
KEY WORDS: Asian clam, antioxidant enzyme, GST, agingDOI: 10.1134/S0006297916030044
Abbreviations: CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GST, glutathione S-transferase; ROS, reactive oxygen species; SOD, superoxide dismutase; totSOD, total superoxide dismutase (MnSOD + CuZnSOD).
Aerobic organisms must deal with continuous production of reactive
oxygen species (ROS) generated during their respiratory activity [1]. These ROS, both physiological messengers and
normal byproducts of cellular metabolism, including superoxide anion
radical (O2•–), hydrogen
peroxide (H2O2), and the highly reactive hydroxyl
radical (OH•), are produced in the mitochondrial
electron transport chain, microsomes of endoplasmic reticulum,
peroxisomes, and the cytosol [2].
Oxidative stress occurs in organisms when ROS generation is transiently or chronically enhanced, and the antioxidant protection system does not counteract the disturbed physiological condition [3]. Excessive ROS can induce deleterious effects on cellular macromolecules such as DNA, proteins, and lipids, which can lead to altered cellular function or cell death [2]. Several studies have suggested that, as an organism ages, ROS naturally begin to accumulate within cells, with ROS levels exceeding capacities of detoxifying antioxidants within the cells [4]. The degree of oxidative stress and oxidative damage is therefore a balance between ROS production and antioxidant defenses [2]. Antioxidants comprise enzymatic and nonenzymatic components. The main antioxidant defense enzymes in all organisms are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), and the phase II biotransformation enzyme glutathione-S-transferase (GST). These enzymes can be induced by ROS, and therefore they can be indicators of oxidative stress [5].
Antioxidant enzyme activity can be influenced by both exogenous and endogenous factors, such as developmental stage, age, diet, seasonality/reproductive cycle, temperature, hypoxia/hyperoxia, and exposure to various types of pollutants [6]. In marine and freshwater organisms, antioxidant enzymes have been widely used as biomarkers of pollution by metals and organic compounds that generate oxidative stress [7]. Previous authors have studied ROS production and oxidative stress mostly in marine bivalves, especially in sentinel organisms used for monitoring environmental conditions [8-10]. On the other hand, there are only a few studies regarding aging in mollusks, especially freshwater species [11, 12]. The aging process in bivalves involves a loss of energetic function and higher susceptibility to oxidative stress followed by progressive impairment of cells, tissues, and organs that is associated with a decline in physiological function over their lifetime [12]. Due to their reduced mobility in post-settlement stages, the age of bivalves is an inevitable factor for determining accumulation, detoxification, adaptive responses, and toxicity of various xenobiotics.
Hence, this research work included the bivalve Corbicula fluminea (O. F. Müller, 1774) inhabiting a location far from large urban and/or industrial settlements, to avoid influence of possible pollutants on antioxidant enzyme activities of the clam. Corbicula fluminea, which has been shown to have potential as a reliable sentinel species [13, 14], was originally distributed in Asiatic ecosystems, bit it is now a common inhabitant of American and European freshwater habitats. The lifespan of C. fluminea varies with habitat, but on average, it ranges from 1 to 5 years [15].
In this regard, this work includes examination of relationships between the age of C. fluminea individuals and the antioxidant enzymatic activity level in order to find differences that might correlate with aging.
MATERIALS AND METHODS
Sampling of C. fluminea and age-class assignment. The Asian clam C. fluminea was collected manually and with an Eckman dredge in October 2010 at the Bela Stena site (44°50′48N, 20°35′12E). Bela Stena, far away from large urban or industrial settlements, is considered as an unpolluted or very low pollution site. Water physicochemical characteristics at the sampling site were determined as temperature (15.9°C), pH (8.1), and dissolved oxygen (9.1 mg/liter). The organisms were stored in glass containers and transported to the laboratory. Specimens were measured for the anterior-posterior (AP) shell length with a Vernier caliper and classified into four size classes according to [16]. Those authors recognized four generations of C. fluminea in the tidal Potomac River (Maryland, USA) with shell size classes of <13 mm (I class), 13 to 18 mm (II class), 19 to 24 mm (III class), and >25 mm (IV class) corresponding to 0+-, 1+-, 2+-, and 3+-year-old individuals, respectively. After determination of age classes, the clams’ bodies were separated from the shells and frozen (–80°C) until further biochemical analysis.
Tissue processing. In each age class (n = 12), the visceral mass was dissected from the collected clams. Visceral masses were ground and homogenized in five volumes of 25 mM sucrose solution containing 10 mM Tris-HCl, pH 7.5, at 4°C [17]. The homogenates were sonicated for 15 s at 10 kHz on ice to release enzymes [18] and then centrifuged in a Beckman ultracentrifuge (90 min, 100,000g, at 4°C). The resulting supernatant fractions were used for further biochemical analyses.
Antioxidant enzymes analyses. Protein concentrations were determined by the Folin phenol reaction as previously described [19]. Total SOD activity was quantified using the epinephrine method [20]. The method is based on the capacity of SOD to inhibit the autoxidation of epinephrine to adrenochrome and is expressed as U/mg protein. CAT activity was evaluated by the rate of H2O2 decomposition [21]. The method is based on H2O2 degradation by the action of CAT contained in the examined samples. CAT activity is expressed as µmol H2O2/min per mg protein. Determination of GPX activity was based on the oxidation of reduced NADPH with t-butylhydroperoxide as a substrate [22]. The enzyme activity is expressed as nmol NADPH/min per mg protein. GR activity was assayed by a published method [23] based on the ability of GR to reduce glutathione disulfide (GSSG) to reduced glutathione (GSH) using NADPH as a substrate. The enzyme activity is expressed as nmol NADPH/min per mg protein. The activity of GST toward 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate was determined according to a published procedure [24]. The method is based on the reaction of CDNB with the -SH group of GSH catalyzed by GST contained in the samples. The enzyme activity is expressed as nmol GSH/min per mg protein.
Statistical analysis. Differences between antioxidant and GST enzymes obtained in each life stage of clams were compared using one-way analysis of variance (ANOVA), followed by the post-hoc Tukey honest significant difference (HSD) multiple-comparison test. The data were first tested for normality (Kolmogorov–Smirnov normality test) and homogeneity of variance (Levene’s test). Results are expressed as mean ± S.D. Correlation analyses were performed within each age-class for all biochemical data using Pearson’s correlation coefficient. Values of P ≤ 0.05 are considered significant. To minimize redundancy, one variable from the pair of highly correlated variables (r > 0.7) are omitted from subsequent analysis. Canonical discriminant analysis (CDA) was performed to classify groups of clams according to the obtained results. All analyses were performed using SAS 9.1.3 software (SAS Institute, Cary, NC, USA).
RESULTS
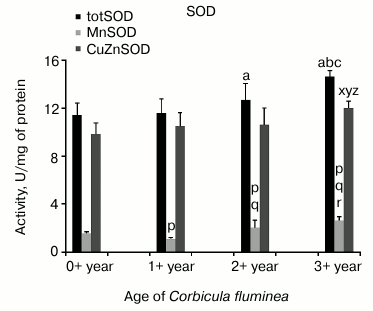
Regarding inter-age comparison of oxidative stress responses, increased levels of totSOD (MnSOD + CuZnSOD) in 2+- and 3+-year-old clams (compared to 0+- and 0+-, 1+-, 2+-year-old individuals, respectively), MnSOD activity in 1+-, 2+-, and 3+-year-old clams (compared to 0+-; 0+- and 1+-; 0+-, 1+-, 2+-year-old individuals, respectively) and CuZnSOD activity in 3+-year-old clams (compared to the other three age classes) were observed (Fig. 1).
Fig. 1. Values represent the means ± SD of totSOD, MnSOD, and CuZnSOD activity measured in the visceral mass of C. fluminea of different age classes (corresponding to the 0+-, 1+-, 2+-, and 3+-year-old clams) collected at Bela Stena locality in the Danube River. The significant differences between classes are: for totSOD: a vs. 0+ year; b vs. 1+ year; c vs. 2+ year; for MnSOD: p vs. 0+ year; q vs. 1+ year; r vs. 2+ year; for CuZnSOD: x vs. 0+ year; y vs. 1+ year; z vs. 2+ year.
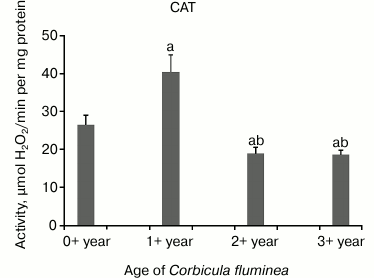
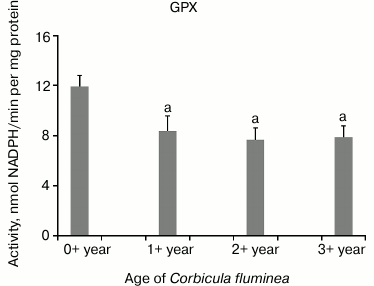
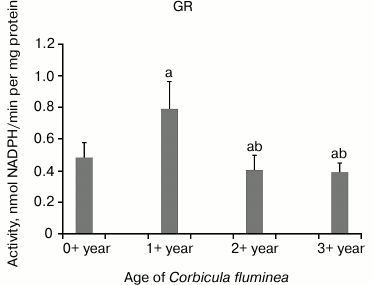
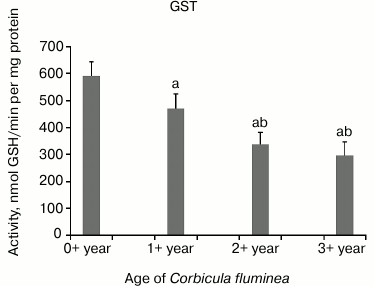
The highest CAT activity was in 1+-year-old clams compared to all the other age-classes, while decreased CAT activity in 2+- and 3+-year-old clams (P < 0.05) was found (Fig. 2). Decreased GPX activity in 1+-, 2+-, and 3+-year-old clams compared to the youngest clams (P < 0.05) was found (Fig. 3). Considering inter-age comparison, GR activity exhibited the same pattern as CAT activity (Fig. 4). GST activity changed in decreasing manner, showing the highest activity in 0+-year-old clams and the lowest activity in 3+-year-old individuals with significant difference at P < 0.05 (Fig. 5).
Fig. 2. Values represent the means ± SD of CAT activity measured in the visceral mass of C. fluminea of different age classes (corresponding to the 0+-, 1+-, 2+-, and 3+-year-old clams) collected at Bela Stena locality in the Danube River. The significant differences between classes are: a vs. 0+ year; b vs. 1+ year; c vs. 2+ year.
Fig. 3. Values represent the means ± SD of GPX activity measured in the visceral mass of C. fluminea of different age classes (corresponding to the 0+-, 1+-, 2+-, and 3+-year-old clams) collected at Bela Stena locality in the Danube River. The significant differences between classes are: a vs. 0+ year; b vs. 1+ year; c vs. 2+ year.
Fig. 4. Values represent the means ± SD of GR activity measured in the visceral mass of C. fluminea of different age classes (corresponding to the 0+-, 1+-, 2+-, and 3+-year-old clams) collected at Bela Stena locality in the Danube River. The significant differences between classes are: a vs. 0+ year; b vs. 1+ year; c vs. 2+ year.
Fig. 5. Values represent the means ± SD of GST activity measured in the visceral mass of C. fluminea of different age classes (corresponding to the 0+-, 1+-, 2+-, and 3+-year-old clams) collected at Bela Stena locality in the Danube River. The significant differences between classes are: a vs. 0+ year; b vs. 1+ year; c vs. 2+ year.
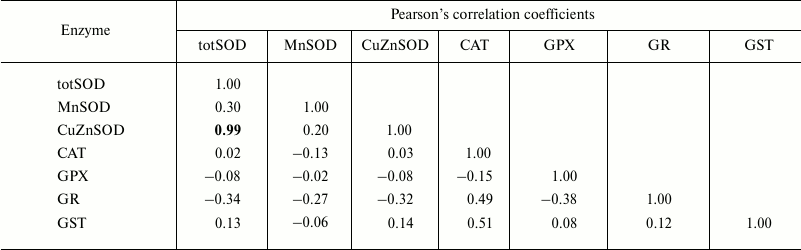
The correlations between the assessed antioxidant parameters were tested within each age class (Tables 1-4). Hence, Pearson’s analysis revealed positive correlations between totSOD activity and the activity of CuZnSOD in all the age classes (Tables 1-4). In addition, totSOD was positively correlated with CAT activity, while CAT activity was positively correlated with GST in 0+-year-old clams (Table 1). Positive correlation was found between CuZnSOD and GPX activity in 3+-year-old clams (Table 4). In all the age classes, MnSOD and GR activities showed no significant correlations with other antioxidant enzymes (Tables 1-4).
Table 1. Pearson’s correlation
coefficients determined, within age class I (0+-year-old clams),
between antioxidant and GST enzymes measured in the visceral mass of
C. fluminea (n = 12 animals per group) collected at
Bela Stena locality in the Danube River
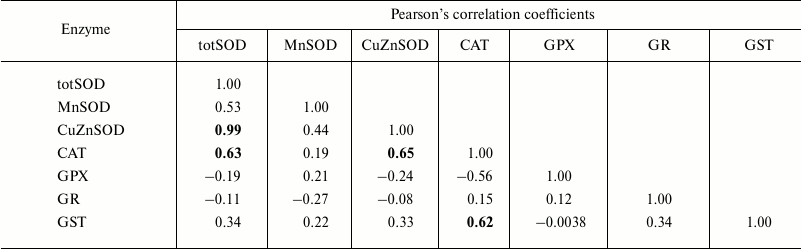
Note: Significant correlations at the 0.05 level are presented in
bold.
Table 2. Pearson’s correlation
coefficients determined, within age class II (1+-year-old clams),
between antioxidant and GST enzymes measured in the visceral mass of
C. fluminea (n = 12 animals per group) collected at
Bela Stena locality in the Danube River
Note: Significant correlations at the 0.05 level are presented in
bold.
Table 3. Pearson’s correlation
coefficients determined, within age class III (2+-year-old clams),
between antioxidant and GST enzymes measured in the visceral mass of
C. fluminea (n = 12 animals per group) collected at
Bela Stena locality in the Danube River
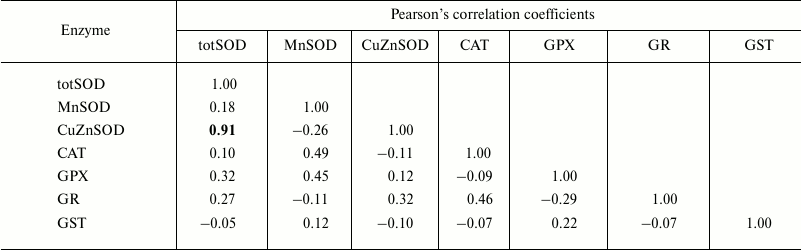
Note: Significant correlations at the 0.05 level are presented in
bold.
Table 4. Pearson’s correlation
coefficients determined, within age class IV (3+-year-old clams),
between antioxidant and GST enzymes measured in the visceral mass of
C. fluminea (n = 12 animals per group) collected at Bela
Stena locality in the Danube River
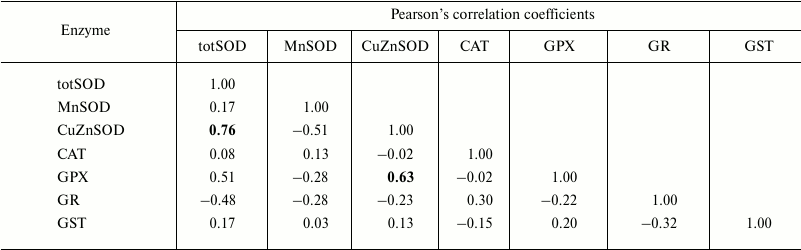
Note: Significant correlations at the 0.05 level are presented in
bold.
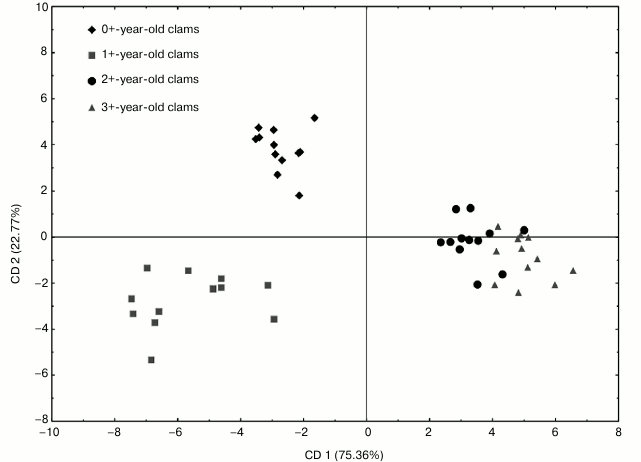
Canonical discriminant analysis (CDA) was performed to discriminate between the age classes of Asian clams according to the measured biochemical parameters and to establish the most important parameter for the separation of groups. The data subjected to CDA analysis were the activities of the antioxidant and GST enzymes measured in the visceral mass of C. fluminea sampled from the Bela Stena locality (Fig. 6).
Fig. 6. Scatterplot of canonical scores representing the four age classes of C. fluminea collected at the Bela Stena locality in the Danube River: age class I (0+-year-old clams), age class II (1+-year-old clams), age class III (2+-year-old clams), and age class IV (3+-year-old clams). The projection was made for all enzyme activities measured in visceral mass of C. fluminea on the factor plane. Groups were formed by CD 1 (the first canonical function) and CD 2 (the second canonical function).
The first two canonical axes explained 98.13% of the overall differences. MnSOD and CAT displayed the highest values of standardized coefficients having the highest contribution to the first axis (CD 1) (Table 5).
Table 5. Standardized coefficients for
canonical variables: antioxidant enzymes and phase II biotransformation
enzyme GST activities measured in the visceral mass of C.
fluminea collected from Bela Stena locality in the Danube River
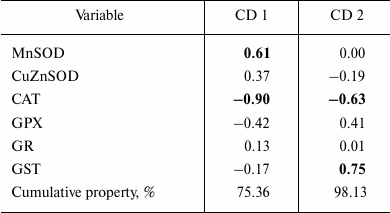
Figure 6 depicts a clear separation along CD 1 for the younger animals (0+- and 1+-year-old ones) that had lower MnSOD and higher CAT activities as compared to the older animals (2+- and 3+-year-old ones) that displayed higher MnSOD and lower CAT activities. Ordination along the second canonical axis (CD 2) revealed segregation of age class I (0+-year-old clams) and age class II (1+-year-old clams) (Fig. 6). The highest contribution to the CD 2 had CAT and GST activities (Table 5), where lower CAT and higher GST activity were measured in animals belonging to age class I (Figs. 2 and 5), and vice versa in age class II animals (Figs. 2 and 5).
DISCUSSION
Some amount of oxidative damage takes place under normal conditions. The rate of this damage increases during the aging process as the result of decreasing efficiency of antioxidant and repair mechanisms. A supportive theme of the oxidative stress hypothesis of aging is that ROS are the primary causal factor underlying aging-associated declines in physiological function [25].
To counteract the toxic effects of ROS, aerobic organisms use a variety of enzymatic and nonenzymatic antioxidants. Antioxidant enzymes form an important protective mechanism against ROS, and their effectiveness may vary with developmental stage and other physiological aspects of the organism [1, 26]. Antioxidant enzymes addressed in this study were totSOD (along with CuZnSOD and MnSOD), CAT, GPX, GR, and phase II biotransformation enzyme, GST.
SOD (EC 1.15.1.1) converts O2•– to H2O2. In eukaryotes, it exists in three different forms: manganese-containing superoxide dismutase – MnSOD (in mitochondria), copper zinc-containing superoxide dismutase – CuZnSOD (in cytosol), and total superoxide dismutase – totSOD (comprised of MnSOD and CuZnSOD). In this study, totSOD and CuZnSOD activities increased with aging, with the exception of MnSOD, which exhibited slightly lower activity in 1+-year-old clams compared to 0+-year-old clams (P < 0.05) with subsequent elevated levels in 2+- and 3+-year-old clams (P < 0.05) (Fig. 1).
Some other authors have found age-related decrease in SOD activity, explaining it with irreversible inactivation by its product, H2O2, as well as with Cu or Zn deficiency [27]. In work [28], a significant reduction in some mitochondrial enzymes with age in short-lived scallop Aequipecten opercularis (life span 8-10 years) was found, and it was suggested that this may represent a decrease in number of mitochondria with age leading to reduced fitness in older individuals. The elevated activity of all three forms of the SOD obtained in older Asian clams could be due to a compensatory mechanism in response to increased generation of O2•– as a function of age and/or a protective mechanism against exogenous O2•– in senescent individuals.
CAT (EC 1.11.1.6) catalyzes the decomposition of H2O2 to water, while GPX (EC 1.11.1.9) detoxifies H2O2 and decomposes organic hydroperoxides to corresponding alcohols. This work revealed higher CAT activity in younger animals with two-fold lower values in older animals compared to 1+-year-olds (Fig. 2), which is probably linked to increased metabolic activity related to the intense reproductive activity that occurs in autumn. In bivalves Crassostrea virginica and Mercenaria mercenaria, SOD did not show a compensatory increase with aging in either species, while CAT significantly decreased with age [29]. SOD and CAT activity increased in the foot tissue of the bivalve Arctica islandica with age. This upward trend of antioxidants with age is rarely observed in ectotherms [30]. The current study showed that GPX activity changed in decreasing manner from the youngest to the oldest clams (Fig. 3), while beside increased SOD, increased GPX with age in the shrimp Aristeus antennatus was noted [31].
The results in this work are concomitant with findings in the marine amphipod Gammarus locusta in which markedly lower GPX in older compared to younger animals was measured [6]. Considering the fact that metabolic rate is normally higher in younger than older clams, the former likely require higher antioxidant and GST activities to protect against potentially increased production of ROS. For bivalves, which are characterized with infinite growth, it is difficult to distinguish between the effects of size and age, which are strongly correlated factors [30].
CAT may play a relatively more important role than GPX in detoxifying H2O2 in invertebrates compared to vertebrates [32]. Studies regarding the effect of aging and size on the activity of CAT in bivalves have given rise to different findings, as this effect appears to be specific to species, sex, tissue, and age. Several authors found a weight-specific decrease in CAT activity within the separate age classes of the mussel Mytilus edulis [33, 34]. Similar results were obtained for other aquatic invertebrates, the marine shrimp A. antennatus [31], as well as for cephalopods Sepia officinalis and Lolliguncula brevis [35]. This finding illustrates that in those studies where age-related decrease of CAT has been reported, it might be due to the increasing size of the aging mussels and not due to the effect of age per se. Considering a much later stage of development and growth, CAT activities and levels of other antioxidants in the mussel M. edulis were lower in older than in younger animals, consistent with lower oxygen consumption rates in the former [33].
GR (EC 1.6.4.2) catalyzes the regeneration of GSH, having a crucial role in preserving normal functioning of other GSH-dependent enzymes. GST (EC 2.5.1.18) catalyzes the conjugation of GSH to electrophilic centers on a wide variety of substrates (endogenous and exogenous) to make the compounds more water-soluble and more easily excreted. On the other hand, the finding of a positive correlation between GPX and GST activities could be explained as coordinated expression of total GST and its peroxidase-like isoform, but reduction of GST activity gained for clams could demonstrate an impairment of GST activity during aging.
The downward trend for CAT, GPX, GR, and GST activities from younger to older Asian clams could be associated with reduction in free radical generation by the mitochondria due to decrease in metabolic rate with increasing size, which may reduce the need for energetically costly production of antioxidant enzymes in larger animals, as observed in A. islandica [30]. This is in agreement with data obtained for the sentinel mussel M. edulis for which has been confirmed a decline in antioxidant capacity with aging [33, 36]. In addition, higher antioxidant activities are seen during an initial phase of active growth in young ocean clams A. islandica, where upon values decline rapidly until the age of maturation and then remain stable [37]. The decreased antioxidant enzyme activities in older clams appear to render the older animals more susceptible to oxidative stress.
Generally, there are few reports describing age-related changes in the activities of antioxidant enzymes in bivalves, especially in freshwater species. Age-related changes in antioxidant and GST enzymes during the life cycle of C. fluminea is expected given that marked alterations in these enzymes activities have been seen in aquatic invertebrates with a number of both endogenous and exogenous factors [26, 38].
Antioxidant enzyme activity can be induced by low concentrations of pollutants and damage under higher concentrations [39]. In relation to pollution and application of antioxidant and GST enzymes as biomarkers in C. fluminea, further investigations focusing on antioxidant defenses versus chemical contamination are now required. Considering the fact that physiological functions in bivalves are influenced by size [34], the contribution of environmental factors as well as ontogenetic development are also determining factors of antioxidant levels in clams [40].
In conclusion, the results in this work show that antioxidant enzyme activities are affected by aging of C. fluminea. Age-related decreases in activities of CAT and glutathione-dependent enzymes were found. On the other hand, significant elevations in totSOD as well as CuZnSOD and MnSOD activities were measured in older clams compared to younger ones. CAT, GPX, GR, and GST activities seem to contribute to antioxidant defense, protecting against oxidative stress, in particular in the younger stages, while totSOD (including CuZnSOD and MnSOD) has more important role in protecting older stages of C. fluminea development.
Antioxidant defense enzymes seem to be balanced with the generation of oxygen-derived species in young individuals; however, there is an increase of oxidative stress in later stages of the Asian clam life cycle. Obtained variations in antioxidant activity levels should be interpreted with adaptive changes in antioxidant enzymes to environmental factors of freshwater natural habitats accompanying aging. Therefore, the aging process of Asian clam remains largely unexplored, and further detailed investigations should be addressed.
This research was supported by the Ministry of Education, Science and Technological Development of Republic of Serbia, Grant No. 173025.
REFERENCES
1.Halliwell, B., and Gutteridge, J. M. C. (1999)
Free Radicals in Biology and Medicine, 3rd Edn., Oxford
University Press, Oxford, U.K.
2.Bodnar, A. G. (2009) Marine invertebrates as models
for aging research, Exp. Gerontol., 44, 477-484.
3.Livingstone, D. R., Garcia-Martinez, P., Michel,
X., Narbonne, J. F., O’Hara, S., Ribera, D., and Winston, G. W.
(1990) Oxyradical production as a pollution-mediated mechanism of
toxicity in the common mussel Mytilus edulis L. and other
molluscs, Funct. Ecol., 4, 415-424.
4.Oberley, T. D., Swanlund, J. M., Zhang, H. J., and
Kregel, K. C. (2008) Aging results in increased autophagy of
mitochondria and protein nitration in rat hepatocytes following heat
stress, J. Histochem. Cytochem., 56, 615-627.
5.Van der Oost, R., Beyer, J., and Vermeulen, N. P.
E. (2003) Fish bioaccumulation and biomarkers in environmental risk
assessment: a review, Environ. Toxicol. Pharmacol., 13,
57-149.
6.Correia, A. D., Costa, M. H., Luis, O. J., and
Livingstone, D. R. (2003) Age-related changes in antioxidant enzyme
activities, fatty acid composition and lipid peroxidation in whole body
Gammarus locusta (Crustacea: Amphipoda), J. Exp. Mar. Biol.
Ecol., 289, 83-101.
7.Pascoli, F., Negrato, E., Di Giancamillo, A.,
Bertotto, D., Domeneghini, C., Simontacchi, C., Mutinelli, F., and
Radaelli, G. (2011) Evaluation of oxidative stress biomarkers in
Zosterisessor ophiocephalus from the Venice Lagoon, Italy,
Aquat. Toxicol., 101, 512-520.
8.Cheung, C. C. C., Zheng, G. J., Li, A. M. Y.,
Richardson, B. J., and Lam, P. K. S. (2001) Relationships between
tissue concentrations of polycyclic aromatic hydrocarbons and
antioxidative responses of marine mussels, Perna viridis,
Aquat. Toxicol., 52, 189-203.
9.Lima, I., Moreira, S. M., Rendon-Von Osten, J.,
Soares, A. M., and Guilhermino, L. (2007) Biochemical responses of the
marine mussel Mytilus galloprovincialis to petrochemical
environmental contamination along the North-Western coast of Portugal,
Chemosphere, 66, 1230-1242.
10.Luchmann, K. H., Mattos, J. J., Siebert, M. N.,
Granucci, N., Dorrington, T. S., Bicego, M. C., Taniguchi, S., Sasaki,
S. T., Daura-Jorge, F. G., and Bainy, A. C. (2011) Biochemical
biomarkers and hydrocarbons concentrations in the mangrove oyster
Crassostrea brasiliana following exposure to diesel fuel
water-accommodated fraction, Aquat. Toxicol., 105,
652-660.
11.Fernández, C., San Miguel, E., and
Fernández-Briera, A. (2009) Superoxide dismutase and catalase:
tissue activities and relation with age in the long-lived species
Margaritifera margaritifera, Biol. Res., 42,
57-68.
12.Philipp, E. E. R., and Abele, D. (2009) Masters
of longevity: lessons from long-lived bivalves – a mini-review,
Gerontology, 56, 55-65.
13.Vranković, J., and Slavić, M.
(2015) Biomarker responses in Corbicula fluminea to the presence
of dioxin-like polychlorinated biphenyls and seasonal changes, Ecol.
Indic., 48, 99-106.
14.Vranković, J. (2015) Environmental impact
on the antioxidant responses in Corbicula fluminea (Bivalvia:
Veneroida: Corbiculidae) from the Danube River, Ital. J. Zool.,
82, 378-386.
15.Sousa, R., Antunes, C., and Guilhermino, L.
(2008) Ecology of the invasive Asian clam Corbicula fluminea
(Müller, 1774) in aquatic ecosystems: an overview, Ann. Limnol.
Int. J. Limnol., 4, 85-94.
16.Dresler, P., and Cory, R. (1980) The Asiatic
clam, Corbicula fluminea (Müller), in the tidal Potomac
River, Maryland, Estuaries, 3, 150-151.
17.Rossi, M. A., Cecchini, G., and Dianzani, M. U.
(1983) Glutathione peroxidase, glutathione reductase and glutathione
transferase in two different hepatomas and in normal liver, IRCS
Med. Sci. Biochem., 11, 805.
18.Takada, Y., Noguchi, T., Okabe, T., and Kayiyoma,
M. (1982) Superoxide dismutase in various tissues from rabbits bearing
the Vx-2 carcinoma in the maxillary sinus, Cancer Res.,
42, 4233-4235.
19.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) Protein measurement with the Folin phenol
reagent, J. Biol. Chem., 193, 265-275.
20.Misra, H. P., and Fridovich, I. (1972) The role
of superoxide anion in the autooxidation of epinephrine and a sample
assay for superoxide dismutase, J. Biol. Chem., 247,
3170-3175.
21.Beutler, E. (1982) Catalase, in Red Cell
Metabolism, a Manual of Biochemical Methods (Beutler, E.,
ed.) Grune and Stratton, New York, pp. 105-106.
22.Tamura, M., Oschino, N., and Chance, B. (1982)
Some characteristics of hydrogen and alkyl-hydroperoxides metabolizing
systems in cardiac tissue, J. Biochem., 92,
1019-1031.
23.Glatzle, D., Vuilleumier, J. P., Weber, F., and
Decker, K. (1974) Glutathione reductase test with whole blood a
convenient procedure for the assessment of the riboflavin status in
humans, Experientia, 30, 665-667.
24.Habig, W. H., Pabst, M. J., and Jakoby, W. B.
(1974) Glutathione S-transferase, J. Biol. Chem., 249,
7130-7139.
25.Kregel, K. C., and Zhang, H. (2007) An integrated
view of oxidative stress in aging: basic mechanisms, functional
effects, and pathological considerations, Am. J. Physiol. Regul.
Integr. Comp. Physiol., 292, 18-36.
26.Livingstone, D. R. (2001) Contaminated-stimulated
reactive oxygen species production and oxidative damage in aquatic
organisms, Mar. Pollut. Bull., 42, 656-666.
27.Inal, M. E., Kanbak, G., and Sunal, E. (2001)
Antioxidant enzyme activities and malondialdehyde levels related to
aging, Clin. Chim. Acta, 305, 75-80.
28.Philipp, E., Brey, T., Heilmayer, O., Abele, D.,
and Pörtner, H. O. (2006) Physiological aging in a polar and a
temperate swimming scallop, Mar. Ecol. Prog. Ser., 307,
187-198.
29.Ivanina, A. V., Sokolova, I. M., and Sukhotin, A.
A. (2008) Oxidative stress and expression of chaperones in aging
mollusks, Comp. Biochem. Physiol., 150, 53-61.
30.Basova, L., Begum, S., Strahl, J., Sukhotin, A.,
Brey, T., Philipp, E., and Abele, D. (2012) Age-dependent patterns of
antioxidants in Arctica islandica from six regionally separate
populations with different life spans, Aquat. Biol., 14,
141-152.
31.Mourente, G., and Diaz-Salvago, E. (1999)
Characterization of antioxidant systems, oxidation status and lipids in
brain of wild-caught size-class distributed Aristeus antennatus
(Risso, 1816) Crustacea, Decapoda, Comp. Biochem. Physiol.,
124, 405-416.
32.Livingstone, D. R., Lips, F.,
Garcia-Martínez, P., and Pipe, R. K. (1992) Antioxidant enzymes
in the digestive gland of the common mussel Mytilus edulis,
Mar. Biol., 112, 265-276.
33.Viarengo, A., Canesi, L., Pertica, M.,
Livingstone, D. R., and Orunesu, M. (1991) Age-related lipid
peroxidation in the digestive gland of mussels: the role of antioxidant
defense systems, Experientia, 47, 454-457.
34.Sukhotin, A. A., Abele, D., and Pörtner, H.
O. (2002) Growth, metabolism and lipid peroxidation in Mytilus
edulis: age and size effects, Mar. Ecol. Prog. Ser.,
226, 223-234.
35.Zielinski, S., and Pörtner, H. O. (2000)
Oxidative stress and antioxidative defense in cephalopods: a function
of metabolic rate or age? Comp. Biochem. Physiol., 125,
147-160.
36.Sukhotin, A. A., and Pörtner, H. O. (2001)
Age-dependence of metabolism in mussels Mytilus edulis (L.) from
the White Sea, J. Exp. Mar. Biol. Ecol., 257, 53-72.
37.Abele, D., Strahl, J., Brey, T., and Philipp, E.
(2008) Imperceptible senescence: aging in the ocean quahog Arctica
islandica, Free Radic. Res., 42, 474-480.
38.Borković, S. S., Šaponjić,
J. S., Pavlović, S. Z., Blagojević, D. P.,
Milošević, S. M., Kovačević, T. B.,
Radojičić, R. M., Spasić, M. B.,
Žikić, R. V., and Saičić, Z. S. (2005) The
activity of antioxidant defense enzymes in the mussel Mytilus
galloprovincialis from the Adriatic Sea, Comp. Biochem.
Physiol., 141, 366-374.
39.Cheng, C. H., Yang, F. F., Ling, R. Z., Liao, S.
A., Miao, Y. T., Ye, C. X., and Wang, A. L. (2015) Effects of ammonia
exposure on apoptosis, oxidative stress and immune response in
pufferfish (Takifugu obscurus), Aquat. Toxicol.,
164, 61-71.
40.Rudneva, I. I. (1999) Antioxidant system of Black
Sea animals in early development, Comp. Biochem. Physiol. C
Pharmacol. Toxicol. Endocrinol., 122, 265-271.